No products in the cart.
Sale
Omramotide (CAS 2306406-71-1) | GMP Supplier & Manufacturer
Original price was: $23.00.$18.00Current price is: $18.00.
Omramotide is an experimental immunological agent for active immunization (antineoplastic), designed to enhance host immune responses against tumor-associated antigens. Manufactured under GMP-certified standards, Omramotide is suitable for preclinical studies in oncology, immunotherapy, and vaccine research. Available in both wholesale and retail formats. For laboratory research use only.
Description
Contents
hide
1.Product Description
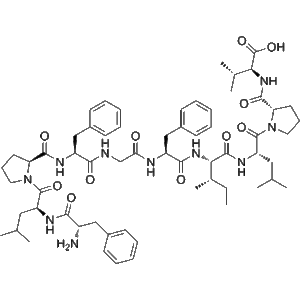
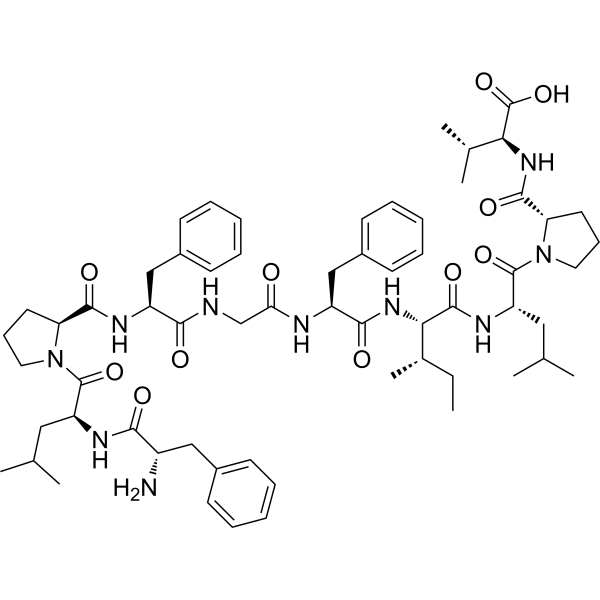
Omramotide (CAS 2306406-71-1) is a synthetic immunological peptide agent investigated for its potential to induce active immunization against cancer-related antigens. As an antineoplastic research compound, Omramotide is part of the growing class of peptide-based immunotherapies designed to harness the immune system for tumor control.
Background
Cancer immunotherapy has emerged as a powerful paradigm in oncology research, aiming to stimulate endogenous immune responses to recognize and eradicate malignant cells. Peptide vaccines such as Omramotide offer a unique advantage by presenting defined antigenic epitopes capable of activating T cells and enhancing adaptive immunity.
Unlike passive immunotherapies (e.g., monoclonal antibodies), Omramotide focuses on active immune training, promoting long-term memory responses. This makes it highly valuable for investigating strategies that achieve durable tumor suppression and reduced recurrence risk.
Research Value
Omramotide provides a controlled and reproducible peptide backbone that allows researchers to:
Analyze T-cell mediated cytotoxicity in tumor models.
Evaluate tumor-specific immune activation without systemic overstimulation.
Study cancer vaccine design principles in preclinical settings.
Compare peptide immunization with checkpoint inhibitors, cytokine therapies, and oncolytic vaccines.
Comparison with Other Immunological Agents
Romurtide: A muramyl dipeptide derivative mainly inducing cytokine release.
Enavermotide: Focused on UCP2-related tumor immune mechanisms.
Omramotide: Specifically optimized as a synthetic peptide immunogen for tumor antigen presentation and T-cell activation.
Advantages
GMP-grade purity ensures reproducible immune responses.
Defined peptide sequence allows precise immunological characterization.
Stability in lyophilized form, ideal for long-term storage and transport.
Compatibility with adjuvants for enhanced immunization effects.
Omramotide supports translational oncology research by providing a standardized peptide agent for active cancer immunization studies.
2. Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Omramotide |
| CAS Number | 2306406-71-1 |
| Molecular Type | Synthetic immunological peptide |
| Category | Antineoplastic agent (immunization research) |
| Synonyms | N/A (unique identifier: Omramotide) |
| Appearance | White to off-white lyophilized powder |
| Purity | 98% (HPLC) |
| Solubility | Soluble in sterile aqueous buffers, DMSO |
| Stability | Stable 24 months in lyophilized form |
| Storage Conditions | Store at -20°C; protect from light; avoid repeated freeze-thaw cycles |
| GMP Compliance | Produced under GMP-certified manufacturing |
| Mechanism | Induces immune activation for antineoplastic research |
| Applications | Cancer immunotherapy, peptide vaccine studies, tumor immunology research |
| Availability | Wholesale & retail supply |
| Safety Considerations | For research use only; not for human or veterinary administration |
| Experimental Models | Murine tumor models, humanized immune system models, in vitro T-cell assays |
Each specification is supported by detailed GMP documentation, ensuring researchers can integrate Omramotide seamlessly into oncology studies.
3. Mechanism of Action & Research Applications
Mechanism of Action
Omramotide functions as a synthetic immunological peptide immunogen, stimulating active immunization through:
Antigen Presentation – Facilitates peptide uptake by antigen-presenting cells (APCs).
T-cell Activation – Promotes MHC class I/II presentation, activating CD8+ cytotoxic and CD4+ helper T cells.
Cytokine Induction – Enhances IFN-?, TNF-?, and IL-2 production, boosting antitumor immunity.
Memory Response Formation – Establishes long-term adaptive immunity against tumor recurrence.
Research Applications
Oncology Models: Evaluating immune-mediated tumor regression in preclinical cancer models.
Vaccine Development: Serving as a template for cancer vaccine adjuvant and delivery system testing.
Tumor Microenvironment Studies: Investigating immune infiltration, T-cell exhaustion, and checkpoint inhibitor synergy.
Combination Therapy: Exploring co-administration with checkpoint inhibitors (anti-PD-1, anti-CTLA-4) to enhance efficacy.
Translational Research: Informing human therapeutic development strategies for peptide-based cancer vaccines.
Omramotide’s versatility makes it a central tool in dissecting immune-oncology pathways and in optimizing cancer immunotherapy protocols.
4. Side Effects (Research Reference)
Preclinical observations of Omramotide in experimental models include:
Local Injection Reactions: Mild redness or swelling at peptide administration sites.
Systemic Cytokine Response: Elevated cytokines may cause transient flu-like symptoms in animal models.
Autoimmune Considerations: Overactivation of immune pathways may mimic autoimmunity in sensitive strains.
Species-Specific Variability: Humanized vs. murine immune systems show different peptide responses.
Stability-Dependent Effects: Degradation products may reduce immunogenicity if storage guidelines are not followed.
These findings are limited to laboratory research. Omramotide is not for human or veterinary use.
5. Disclaimer
For laboratory research use only. Not for human or veterinary use.
6. Keywords
- CAS 2306406-71-1
Cancer immunization peptide
Antineoplastic immunological agent
GMP supplier Omramotide
Tumor immunotherapy research peptide
Active immunization peptide cancer vaccine
Laboratory oncology research peptide
Preclinical cancer vaccine peptide
Immunological peptide for research
Additional information
| Weight | 0.9 kg |
|---|---|
| Dimensions | 83 × 52 × 83 cm |
What is Omramotide?
An immunological peptide agent for active cancer immunization research.
What is its CAS number?
CAS No. 2306406-71-1.
What category does Omramotide belong to?
Antineoplastic agent for immunological research.
Is it GMP-certified?
Yes, manufactured under GMP compliance.















Reviews
There are no reviews yet.