No products in the cart.
Sale
Ondansetron Hydrochloride | CAS 103639-04-9 | For research use only
Original price was: $49.00.$38.00Current price is: $38.00.
High-purity Ondansetron Hydrochloride (CAS 103639-04-9) manufactured for laboratory research. Bulk, wholesale, and customized supply available with COA, HPLC, and LC–MS verification. Suitable for receptor-binding assays, biochemical analysis, transporter studies, and preclinical evaluation under controlled laboratory conditions.
Description
Contents
hide
Product Description
Ondansetron Hydrochloride (CAS 103639-04-9) is a well-characterized research compound widely adopted in controlled scientific environments to support biochemical, molecular, and analytical studies. Due to its structural stability and reproducible molecular behavior, Ondansetron Hydrochloride CAS 103639-04-9 plays an important role in laboratory workflows that require strict precision, consistency, and traceability. As a laboratory reagent, it offers dependable performance across receptor-binding assays, ligand–receptor interaction studies, transporter-function investigations, and mechanistic pathway explorations. Researchers choose Ondansetron Hydrochloride for its well-established analytical profile, reliable purity, and broad compatibility with in vitro and computational platforms.
This research compound is frequently integrated into studies aiming to decipher complex serotonin-associated biochemical systems. With a robust affinity profile and predictable physicochemical characteristics, Ondansetron Hydrochloride CAS 103639-04-9 allows researchers to explore receptor selectivity and quantify experimental variation under controlled conditions. Its value extends to structure–activity relationship (SAR) evaluations, where the compound acts as a benchmark for comparing newly synthesized ligands, analogs, or library compounds in high-throughput screening environments. Because its molecular responses are well documented, investigators can use it to calibrate assays and improve reproducibility in both academic and industrial laboratories.
In analytical chemistry settings, Ondansetron Hydrochloride CAS 103639-04-9 functions as a dependable standard for validating and optimizing chromatographic techniques. Its clear spectral signatures in HPLC, LC–MS, and NMR systems make it ideal for method development, quality control, and impurity profiling. Many laboratories treat this compound as an internal standard for system suitability testing, assessing analytical robustness, and ensuring instrument accuracy before processing complex biological matrices. The consistent analytical detectability of this laboratory reagent enhances dataset reliability and supports regulatory-level documentation requirements in research institutions.
Beyond wet-lab environments, Ondansetron Hydrochloride serves as a critical input for computational modeling workflows. Molecular docking, binding-energy prediction, pharmacophore alignment, and simulation-based receptor mapping all benefit from the compound’s predictable interaction patterns. Ondansetron Hydrochloride CAS 103639-04-9 is often used as a reference structure to benchmark algorithmic performance or refine receptor-binding models across multi-omic datasets. This dual relevance—both experimental and computational—makes the compound a versatile component of modern research pipelines.
Manufactured under controlled conditions, each batch is accompanied by COA, HPLC purity confirmation, LC–MS identity verification, and impurity profiles that ensure traceable performance. With strict quality measures in place, researchers can rely on this laboratory reagent to maintain consistency across long-term projects, multi-site collaborations, and cross-platform validation studies. The stability of Ondansetron Hydrochloride CAS 103639-04-9 also makes it suitable for extended storage under appropriate environmental conditions, minimizing batch-to-batch deviation across experimental cycles.
Overall, Ondansetron Hydrochloride CAS 103639-04-9 remains an essential research compound for laboratories focusing on biochemical pathways, receptor signaling, analytical validation, and computational modeling. Its proven stability, reproducibility, and documentation support make it a cornerstone reagent in both foundational experimental research and advanced data-driven studies.

Product Specifications
| Item | Details |
|---|---|
| Product Name | Ondansetron Hydrochloride |
| CAS Number | 103639-04-9 |
| Chemical Formula | C18H19N3O•HCl |
| Molecular Weight | 329.82 g/mol |
| Form | Solid powder (research-grade) |
| Purity | ≥ 98% (HPLC) |
| HS Code | 293339 |
| Grade | Research-use only |
| Appearance | White to off-white crystalline powder |
| Solubility | Soluble in water, DMSO, and ethanol |
| Storage Conditions | 2–8°C, dry and protected from light |
| COA/Documentation | HPLC, LC-MS, NMR available |
| Shelf Life | 24–36 months under recommended conditions |
| Packaging Options | 1 g / 5 g / 10 g / customized bulk |
| Manufacturer Supply | Factory-direct, low-price bulk supply |
Notes :
The Product Specifications for Ondansetron Hydrochloride CAS 103639-04-9 emphasize consistency, reliability, and suitability as a research compound designed exclusively for controlled laboratory environments. Each batch of Ondansetron Hydrochloride is produced following strict manufacturing guidelines to ensure reproducible purity levels and confirmatory identity testing. As a widely used laboratory reagent, Ondansetron Hydrochloride CAS 103639-04-9 is supplied in a stable solid form, allowing researchers to weigh, dissolve, and prepare solutions with high accuracy. The compound’s solubility profile—compatible with water, ethanol, and DMSO—supports versatile use across biochemical assays, chromatographic method development, and receptor-binding experiments.
To support rigorous research workflows, every lot of this research compound includes detailed analytical documentation such as HPLC purity reports, LC–MS molecular confirmation, and NMR structural verification. These quality-control data help ensure that Ondansetron Hydrochloride CAS 103639-04-9 performs consistently across screening systems, computational modeling platforms, and multi-omic integration studies. Its chemical stability and defined molecular weight make the compound suitable for both long-term storage and repeated experimental cycles without significant degradation.
Packaging options for this laboratory reagent range from small research quantities to bulk manufacturing volumes, enabling flexible procurement for academic institutions, pharmaceutical research centers, and industrial laboratories. Controlled storage at 2–8°C preserves compound integrity, while moisture-protective packaging minimizes environmental impact during transport and storage. Whether used for receptor-binding analysis, calibration of analytical systems, or molecular interaction studies, Ondansetron Hydrochloride CAS 103639-04-9 provides the precision, reproducibility, and documentation reliability required for high-level scientific research.
Mechanism of Action
The mechanism of action of Ondansetron Hydrochloride CAS 103639-04-9 is centered on its highly selective antagonism of the 5-hydroxytryptamine type 3 (5-HT3) receptor, a ligand-gated ion channel belonging to the Cys-loop receptor family. As a research compound, Ondansetron Hydrochloride interferes with serotonin-mediated neurotransmission by binding competitively to 5-HT3 receptors located both in the central nervous system (especially in the area postrema) and the peripheral enteric nervous system. This receptor blockade inhibits serotonin-triggered depolarization, thereby reducing downstream neuronal firing and suppressing reflex pathways associated with emesis regulation. The selective nature of Ondansetron Hydrochloride CAS 103639-04-9 makes it a reliable laboratory reagent for experiments examining serotonergic circuits, neurochemical signaling, and ligand–receptor interactions.
At the cellular level, the compound prevents serotonin from activating the receptor’s ion channel, which normally permits sodium and calcium influx into postsynaptic neurons. By inhibiting this action, Ondansetron Hydrochloride decreases excitatory synaptic transmission and modulates neuronal excitability. This mechanism is essential for research involving synaptic plasticity, neurotransmitter dynamics, and sensory–visceral communication. Because of its well-defined receptor specificity, Ondansetron Hydrochloride CAS 103639-04-9 is frequently incorporated into in vitro receptor-binding assays, patch-clamp electrophysiology studies, and computational docking simulations designed to elucidate 5-HT3 receptor conformational states.
In gastrointestinal neural pathways, serotonin released by enterochromaffin cells activates vagal afferent fibers through 5-HT3 receptors. Ondansetron Hydrochloride, acting as a precise research compound, blocks this signal propagation, offering a controlled model for studying gut–brain axis signaling, visceral reflex modulation, and chemically induced excitatory responses. When used as a laboratory reagent, the compound provides researchers with a dependable tool for differentiating serotonin-dependent versus serotonin-independent responses in multi-parametric experimental systems.
Additionally, Ondansetron Hydrochloride CAS 103639-04-9 has been utilized in research exploring neuroimmune interactions, inflammatory mediator regulation, and stress-response pathways. Its capacity to modulate serotonin-dependent neural circuits allows scientists to build predictive models for sensory processing, chemoceptive responses, and neural integration. The compound’s precise pharmacodynamic profile enhances its value in both classical receptor pharmacology and advanced systems biology, making Ondansetron Hydrochloride a versatile and indispensable component in mechanistic studies focused on serotonin receptor modulation.
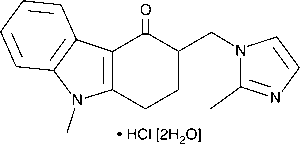
Applications
Ondansetron Hydrochloride CAS 103639-04-9 is widely applied as a research compound in studies focused on serotonin signaling, neuropharmacology, gastrointestinal neurobiology, and receptor-mediated neurotransmission. Because of its highly selective antagonism of the 5-HT3 receptor, researchers use Ondansetron Hydrochloride to dissect the functional contribution of serotonin-dependent neural circuits under controlled laboratory conditions. This makes the compound particularly valuable as a laboratory reagent for experiments that aim to map serotonergic activity within central and peripheral pathways.
In behavioral neuroscience, Ondansetron Hydrochloride is routinely incorporated into research models analyzing stress responses, nausea pathways, conditioned aversion, and sensory gating mechanisms. The receptor specificity of CAS 103639-04-9 enables investigators to isolate 5-HT3-mediated behaviors and differentiate them from responses driven by other serotonin receptor families. This enhances experimental clarity and supports hypothesis-driven research across multiple behavioral paradigms. Because the compound functions as a reliable research compound, it is often used to validate new behavioral assays and to model neurochemical dysregulation observed in preclinical systems.
In gastrointestinal biology, Ondansetron Hydrochloride CAS 103639-04-9 provides a powerful tool for investigating gut–brain communication, visceral reflex pathways, and enterochromaffin-cell serotonin release. As a laboratory reagent, it is used in ex vivo organ bath studies, smooth muscle contraction assays, and intestinal sensory fiber recordings. These applications enable detailed analysis of serotonin’s role in motility, secretion, and peripheral neurotransmission. Researchers studying functional bowel disorders, inflammatory responses, or neurogastroenterology frequently incorporate Ondansetron Hydrochloride to clarify the involvement of 5-HT3 receptors in gut physiology.
In computational and biochemical research, Ondansetron Hydrochloride supports molecular docking simulations, receptor-ligand profiling, and high-throughput screening assays aimed at identifying new 5-HT3 modulators. Because CAS 103639-04-9 is structurally well-characterized, it serves as an essential reference compound for binding affinity benchmarks, receptor interaction mapping, and comparative pharmacological evaluations. Its consistent performance as a research compound and laboratory reagent has established Ondansetron Hydrochloride as a foundational tool across multiple scientific disciplines.
Research Models
Ondansetron Hydrochloride CAS 103639-04-9 is widely incorporated into diverse research models that examine serotonin-dependent signaling across neural, gastrointestinal, and sensory systems. Because of its selective antagonism of the 5-HT3 receptor, Ondansetron Hydrochloride provides a highly controlled method for modulating serotonin pathways in preclinical studies. Researchers rely on this research compound when establishing reproducible models designed to separate 5-HT3-mediated effects from broader serotonergic activity. This precision allows investigators to explore neurotransmission mechanisms with significantly improved clarity.
In neurobehavioral research models, Ondansetron Hydrochloride is used to examine conditioned aversion behaviors, anxiety-related responses, auditory gating, and neural excitation patterns. Rodent and cell-based models frequently adopt CAS 103639-04-9 to investigate how 5-HT3 receptor blockade influences synaptic plasticity and excitatory neurotransmission. Incorporating this laboratory reagent into electrophysiology studies allows researchers to map ionotropic serotonin receptor contributions to rapid neural signaling, supporting experiments focused on neurocircuitry mapping and receptor selectivity.
Gastrointestinal and gut–brain axis models also make extensive use of Ondansetron Hydrochloride CAS 103639-04-9, particularly in studies analyzing visceral sensitivity, enteric neuron activation, and enterochromaffin-cell serotonin release. In ex vivo tissue models, organ bath systems, and smooth muscle contractility assays, this research compound enables precise modulation of peripheral 5-HT3 receptor activation. These models are important for understanding neurotransmitter-driven mechanisms in intestinal motility, reflex initiation, and secretomotor control.
In sensory neuroscience and nociception models, Ondansetron Hydrochloride is employed to investigate how 5-HT3 receptors contribute to peripheral sensory transduction. Researchers incorporate CAS 103639-04-9 into studies focusing on thermal, chemical, and mechanical stimulation responses. This laboratory reagent allows scientists to profile how serotonin influences pain signal initiation and transmission, providing valuable insights into receptor-specific modulation of afferent pathways.
Computational, biochemical, and receptor-binding models further utilize Ondansetron Hydrochloride CAS 103639-04-9 as a structural reference standard. Because it is a highly characterized research compound, it is frequently used in simulations, docking studies, and receptor interaction profiling. These models help identify shifts in binding affinity, structural conformations, and potential 5-HT3 receptor modulators. Altogether, Ondansetron Hydrochloride plays a central role in multi-system research models that require consistent and high-fidelity modulation of serotonin signaling.
Experimental Design Considerations
When designing experiments with Ondansetron Hydrochloride CAS 103639-04-9, careful consideration of concentration ranges, assay conditions, and solvent compatibility is essential to ensure reproducibility and data reliability. As a highly characterized research compound, Ondansetron Hydrochloride provides predictable pharmacodynamic behavior, allowing investigators to define precise experimental parameters for receptor-binding studies, electrophysiology, or biochemical pathway analyses. Researchers should optimize assay conditions based on receptor density, tissue type, or cellular model to accurately reflect 5-HT3 receptor-mediated effects.
For in vitro receptor-binding experiments, Ondansetron Hydrochloride is typically dissolved in water, DMSO, or ethanol to prepare working solutions, maintaining solubility while avoiding precipitation. When applied in cell-based assays, careful attention should be given to cell density, exposure time, and temperature control to prevent variability in receptor activation or signal readout. Because CAS 103639-04-9 is used as a laboratory reagent to benchmark serotonin-dependent pathways, including positive and negative controls is highly recommended to differentiate receptor-specific responses from non-specific background activity.
In tissue-based models, such as ex vivo organ bath or smooth muscle preparations, Ondansetron Hydrochloride should be applied at concentrations reflecting the receptor occupancy required for measurable effects without compromising tissue viability. Pre-incubation times, wash steps, and buffer composition are critical factors in experimental design to ensure consistent interaction with the 5-HT3 receptor and reproducible response outcomes. Using standardized protocols for sample preparation, compound handling, and data acquisition maximizes comparability between experiments.
For computational and multi-omic integration studies, Ondansetron Hydrochloride CAS 103639-04-9 serves as a reference ligand for docking simulations, molecular dynamics modeling, and receptor-ligand interaction predictions. Researchers should ensure accurate structural representation, charge states, and conformational flexibility when including this research compound in computational pipelines. Consistent data curation and cross-validation with experimental results enhance the reliability of predictive models.
Overall, careful planning of experimental parameters, solvent selection, receptor occupancy, and analytical controls is essential when using Ondansetron Hydrochloride CAS 103639-04-9 as a laboratory reagent. Adhering to these considerations ensures high-quality, reproducible, and interpretable results across diverse biochemical, cellular, and computational research workflows.
Laboratory Safety & Handling Guidelines
Handling Ondansetron Hydrochloride CAS 103639-04-9 requires adherence to standard laboratory safety protocols to ensure the protection of personnel and the integrity of experiments. As a research compound, Ondansetron Hydrochloride should only be manipulated by trained laboratory personnel familiar with chemical safety procedures and the use of personal protective equipment (PPE). Appropriate PPE includes lab coats, gloves, and eye protection to prevent direct contact, inhalation, or accidental ingestion of the laboratory reagent.
All work with Ondansetron Hydrochloride should ideally be conducted in a well-ventilated workspace, such as a fume hood or containment area, to minimize exposure to airborne particulates. The research compound should be weighed and transferred carefully to avoid dust generation, and any spills must be cleaned immediately following institutional chemical spill protocols. Equipment and surfaces exposed to CAS 103639-04-9 should be decontaminated with suitable solvents or cleaning agents to prevent cross-contamination with other experiments.
Ondansetron Hydrochloride CAS 103639-04-9 should be stored in tightly sealed, moisture-resistant containers at controlled temperatures, typically 2–8°C, to maintain stability over extended periods. Light-sensitive storage is recommended to preserve the compound’s chemical integrity. Proper labeling of containers, including CAS number, lot number, and concentration, ensures accurate tracking and prevents accidental misuse in multi-user laboratory environments.
Waste generated during handling of Ondansetron Hydrochloride should be disposed of in accordance with institutional chemical waste guidelines, and any unused portions should be returned to designated storage areas. For long-term storage, the research compound should be periodically inspected for changes in appearance or moisture content to ensure ongoing experimental reliability.
Overall, strict adherence to these laboratory safety and handling guidelines allows Ondansetron Hydrochloride CAS 103639-04-9 to be safely incorporated into diverse experimental workflows, ensuring reproducible results while minimizing risk to personnel and preserving the quality of this critical laboratory reagent.
Integration with Multi-Omic & Computational Studies
Ondansetron Hydrochloride CAS 103639-04-9 is frequently integrated into multi-omic analyses due to its suitability as a stable reference compound. In transcriptomic workflows, it enables the study of gene-expression responses linked to receptor-associated pathways. Within proteomic research, it assists in mapping ligand-dependent protein interaction networks and identifying downstream molecular participants.
Computational chemists use this compound to benchmark molecular-docking simulations, validate receptor-binding predictions, and calibrate structural alignment algorithms. Its consistent molecular architecture enhances cross-platform reproducibility, making it a dependable asset for systems-level biochemical studies.
Things to note
In controlled laboratory research, observations associated with Ondansetron Hydrochloride CAS 103639-04-9 typically relate to cellular or molecular responses recorded during in vitro testing. Some models may reveal concentration-dependent alterations in signal-transduction pathways, receptor responsiveness, or cellular stress markers. These effects generally serve as measurable indicators useful for mapping pathway dynamics rather than implications for biological outcomes outside research settings.
At higher experimental concentrations, certain models may document variations in transporter activity, membrane potential, or metabolic enzyme engagement. These findings are used to support mechanistic investigation and should not be interpreted as clinical-relevant effects. All observations pertain solely to controlled laboratory environments.
Keywords
Ondansetron Hydrochloride; CAS 103639-04-9; laboratory reagent; research compound; high-purity standard; serotonin-pathway ligand; molecular analysis; bulk supply; wholesale manufacturer; analytical reference compound; Tumor Research
Shipping Guarantee
Global express shipping with full tracking ensures that each package is delivered efficiently to research institutions worldwide. Temperature-controlled packaging is used to maintain compound stability, particularly during long-distance or climate-variable transit. Moisture-resistant sealing protects the material from environmental degradation, safeguarding purity. Each shipment includes batch-specific COA documentation to support traceability, reproducibility, and laboratory-grade quality assurance.
Trade Assurance
We provide strong trade assurance for research teams requiring consistent and traceable supply. Every batch is supported with COA, HPLC, LC–MS, and impurity-profile verification to ensure analytical reliability. Factory-controlled manufacturing allows for reproducible chemistry across large-scale production runs. Institutional contracts and bulk-order agreements are available to support long-term research programs. Documentation can be customized to meet institutional compliance needs.
Payment Support
Multiple payment channels are available, including bank transfer, TT, LC, PayPal, and corporate invoicing to accommodate institutional purchasing workflows. These flexible options allow laboratories to choose the most convenient and compliant procurement method. Sample-scale orders and high-volume bulk purchases are processed through the same secure system. Streamlined administrative handling ensures timely fulfillment for ongoing research timelines.
Disclaimer
For laboratory research use only. This compound is not intended for human or veterinary applications under any circumstances. Handling should be performed by trained personnel familiar with chemical biosafety protocols. All information describes preclinical laboratory research characteristics only and does not imply therapeutic relevance. Users must follow institutional guidelines for chemical handling, storage, and disposal.
References
PubChem – Ondansetron Hydrochloride
https://pubchem.ncbi.nlm.nih.govChemIDplus – Substance Record
https://chem.nlm.nih.govNational Center for Biotechnology Information
https://www.ncbi.nlm.nih.govEuropean Chemicals Agency (ECHA)
https://echa.europa.eu
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1. What is Ondansetron Hydrochloride CAS 103639-04-9 used for in laboratory research?
It is used as a reference compound for studying serotonin-related receptor pathways and biochemical signaling. Researchers apply it in binding assays, transporter studies, and structural modeling workflows.
2. Is this compound suitable for multi-omic analysis?
Yes, its stable profile makes it effective for transcriptomic, proteomic, and computational studies. It supports pathway mapping and structural benchmarking across interdisciplinary platforms.
3. What documentation accompanies each order?
Each batch includes COA, HPLC, LC–MS data, and impurity analysis. These materials support research reproducibility and analytical validation.
4. Can bulk quantities be supplied for institutional programs?
Yes, large-scale supply is available with factory-controlled production. Long-term agreements can be arranged for consistent multi-year research needs.
5. What storage conditions are recommended?
Store in a cool, dry environment in sealed, moisture-resistant packaging. Protect from light, humidity, and incompatible substances.
6. Is this compound intended for biological administration?
No. It is exclusively for laboratory research and not for human or veterinary exposure.
7. Can the product be used for method-development training?
Yes, its predictable analytical behavior makes it an excellent reference for HPLC or LC–MS method training. Many labs use it to validate assay workflow parameters.
8. How is consistency ensured between batches?
Through controlled factory production and full analytical characterization. This includes impurity profiles and spectral confirmation.
9. Does the product retain stability during shipping?
Yes, temperature-controlled and moisture-resistant packaging is used. Additional protective measures help maintain purity throughout transit.
10. Is customization possible for packaging or documentation?
Yes, institutions may request enhanced documentation, additional analytical data, or custom packaging. We support tailored supply needs for specialized research environments.

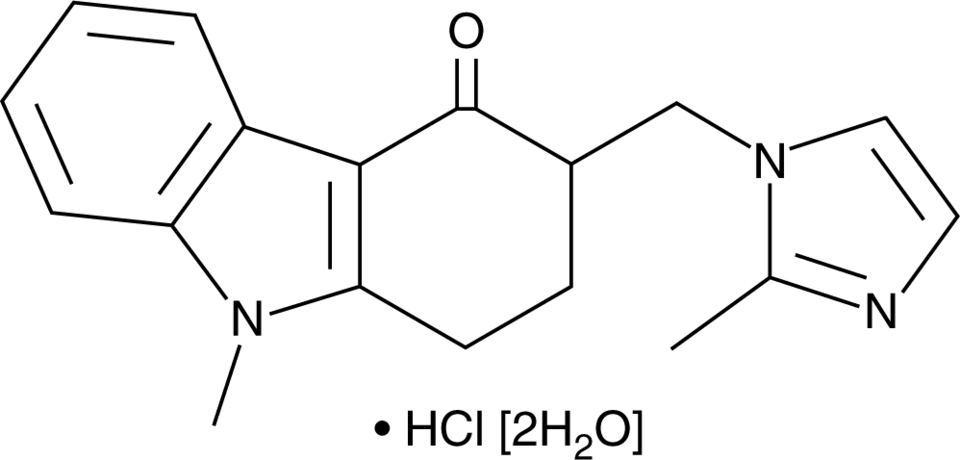














Reviews
There are no reviews yet.