No products in the cart.
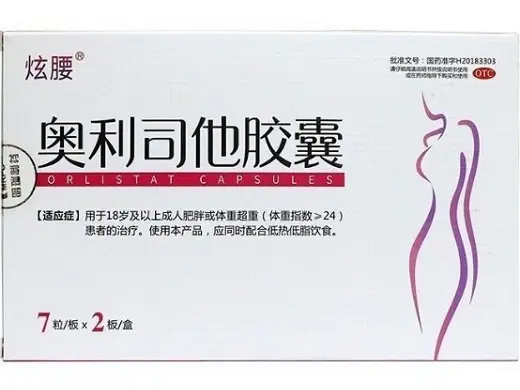
Orlistat Capsules?14 Capsules (XuanYao) Wholesale price comparison
$6.00
Orlistat Capsules (brand name: XuanYao) contain 60?mg of orlistat per capsule and are designed for non-clinical laboratory research in metabolic and gastrointestinal studies. Each box includes 14 capsules (7 capsules × 2 blisters). Manufactured by Hunan Mingrui Pharmaceutical Co., Ltd., with approval number H20183303.?? Strictly for laboratory research use only.?Please consult staff for other specifications and uses?
Description
Orlistat Capsules?XuanYao Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Orlistat Capsules (XuanYao) |
| Generic Name | Orlistat |
| Synonyms | Tetrahydrolipstatin, Xenical (reference brand) |
| Formulation | Oral capsule |
| Strength | 60?mg per capsule |
| Pack Size | 14 capsules (7 capsules × 2 blisters) per box |
| Dosage Form | Capsule |
| CAS Number | 96829-58-2 |
| Approval Number | H20183303 (China NMPA) |
| Product Code | 86905001000182 |
| Manufacturer | Hunan Mingrui Pharmaceutical Co., Ltd. |
| Barcode | 6920945610307 |
| Storage | Store below 25?°C in a cool, dry place |
| Intended Use | For laboratory research only |
Orlistat Capsules?XuanYao Mechanism of Action
Orlistat is a gastrointestinal lipase inhibitor that prevents the enzymatic breakdown of dietary fats in the intestine. By blocking lipase activity, it reduces fat absorption and facilitates excretion. It is widely used in experimental models of obesity, fat metabolism, and gastrointestinal function.
Research Applications & Pharmacology
Preclinical obesity models
Lipid digestion and absorption studies
Bioavailability of fat-soluble vitamins
Drug–nutrient interaction experiments
Low systemic absorption (<1%)
Excreted mainly through feces
Safety Profile & Handling
Reported Preclinical Effects:
GI side effects: steatorrhea, flatulence
Malabsorption of vitamins A, D, E, K
Hepatic changes in some animal models
Lab Handling Instructions:
Wear gloves, eye protection, and a lab coat
Avoid direct contact or inhalation of capsule content
Store in original packaging, away from heat or moisture
Dispose of per hazardous lab waste protocols
?? Research Use Disclaimer
This product is intended strictly for laboratory research purposes and is not approved for therapeutic, diagnostic, or clinical use in humans or animals. Handling must comply with local research safety standards.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 20 × 26 × 20 cm |
Q1: Is this product intended for medical treatment?
A1: No. It is not for human or animal use. Laboratory research only.
Q2: What are common research uses?
A2: Models of diet-induced obesity, fat metabolism, gastrointestinal enzyme inhibition, and nutrient absorption studies.
Q3: Do you provide documentation?
A3: Yes. COA (Certificate of Analysis) and SDS (Safety Data Sheet) are available upon request.
Q4: Do you provide wholesale pricing or OEM service?
A4: Yes. We support bulk orders, OEM production, and custom labeling for qualified partners.
Q5: How should the product be stored in a lab environment?
A5: Store below 25°C, away from moisture, light, and heat.










Reviews
There are no reviews yet.