No products in the cart.
Sale
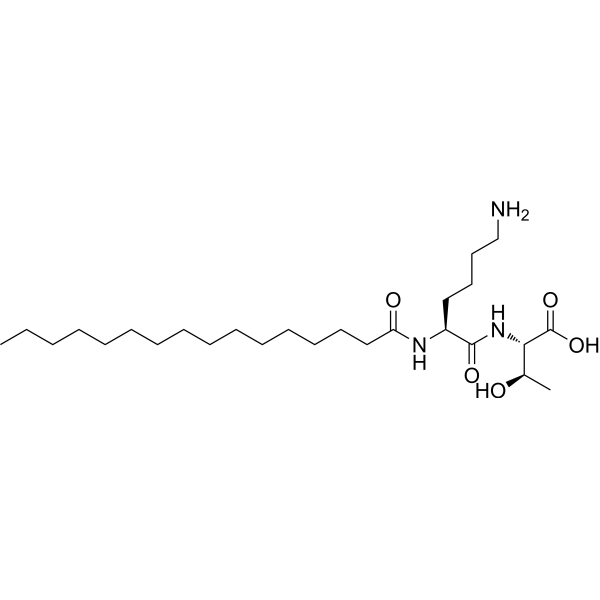
Palmitoyl dipeptide-7 | CAS 911813-90-6 | Anti-Aging Peptide for Collagen Renewal and Skin Firmness
Original price was: $3.00.$2.00Current price is: $2.00.
Palmitoyl dipeptide-7 is a bioactive cosmetic peptide known for its role in enhancing collagen renewal, improving skin elasticity, and reducing visible wrinkles. It functions as a biomimetic peptide that supports extracellular matrix stability and promotes dermal repair, making it widely studied in anti-aging and skin-firmness research applications.
Description
Product Description
Palmitoyl dipeptide-7 is a scientifically designed lipopeptide that integrates a short peptide sequence with a palmitoyl chain to increase its dermal penetration and bioavailability. This hybrid structure allows the molecule to interact directly with skin cells, particularly dermal fibroblasts, which are responsible for producing collagen, elastin, and glycosaminoglycans essential for maintaining skin elasticity and firmness. In cosmetic and dermatological research, palmitoyl dipeptide-7 has been recognized as a potent signal peptide that mimics the natural cellular communication mechanisms involved in tissue repair and regeneration.
This dipeptide’s amphiphilic structure enhances its affinity for lipid membranes, improving stability and transdermal delivery compared to non-lipidated peptides. Studies in vitro have shown that palmitoyl dipeptide-7 can upregulate type I and type III collagen synthesis while also stimulating hyaluronic acid production. These effects are particularly valuable in anti-aging research where the loss of dermal matrix components is a key factor contributing to wrinkle formation and skin sagging.
From a biochemical standpoint, palmitoyl dipeptide-7 operates as a cell-communicating ingredient, transmitting specific peptide signals that instruct fibroblasts to restore extracellular matrix homeostasis. It may also play a role in reducing the expression of matrix metalloproteinases (MMPs), enzymes responsible for collagen degradation, thereby preserving structural integrity within the dermis. This dual mechanism—stimulating synthesis and reducing degradation—makes it a cornerstone compound in modern anti-aging peptide formulations.
In addition to its regenerative effects, palmitoyl dipeptide-7 has demonstrated antioxidative potential by mitigating oxidative stress induced by UV radiation and environmental pollutants. By helping to balance redox activity within skin cells, it contributes to improved cellular longevity and resistance to premature aging. The peptide’s soothing and barrier-supportive attributes also make it relevant in research focused on sensitive or damaged skin.
In cosmetic peptide development, palmitoyl dipeptide-7 is often used synergistically with other matrikines and growth factor analogues to amplify dermal renewal. Researchers have explored its application in topical formulations, encapsulated systems, and controlled-release delivery vehicles to optimize stability and bioactivity. Its compatibility with both aqueous and lipid environments allows flexibility in formulation research across serums, emulsions, and advanced dermal delivery models.
Overall, palmitoyl dipeptide-7 stands out as a next-generation cosmetic peptide that bridges structural biology and biomimetic design. Its relevance spans studies in collagen biosynthesis, fibroblast signaling, extracellular matrix reconstruction, and skin biome resilience—making it a vital molecule in anti-aging and firmness research domains.

Product Specifications
| Item | Description |
|---|---|
| Product Name | Palmitoyl dipeptide-7 |
| CAS Number | 911813-90-6 |
| Molecular Formula | C₃₁H₅₇N₅O₅ |
| Molecular Weight | 595.82 g/mol |
| Appearance | White to off-white powder |
| Purity | ≥99% |
| Solubility | Soluble in water and ethanol |
| Storage Temperature | 2–8 °C |
| Category | Cosmetic Peptide |
| Synonyms | Palmitoyl-Lys-His, Palmitoyl Dipeptide |
| Applications | Collagen renewal, skin firmness, anti-aging, dermal matrix repair research |
| Research Area | Skin biology, peptide biochemistry, cosmetic material science |
| Intended Use | For laboratory research use only |
Mechanism of Action
Palmitoyl dipeptide-7 operates primarily as a matrikine-like signal peptide, emulating the natural fragments of extracellular matrix proteins that regulate cellular communication and regeneration. The peptide’s palmitoyl lipid chain improves its affinity for the stratum corneum, facilitating efficient penetration into the dermal layer, where it can modulate fibroblast activity.
At the cellular level, palmitoyl dipeptide-7 engages with fibroblast membrane receptors, particularly those associated with integrin and TGF-β signaling pathways. This activation cascade leads to enhanced expression of genes responsible for the synthesis of structural proteins such as collagen I, III, and fibronectin. The peptide also helps restore homeostatic balance between collagen synthesis and breakdown by inhibiting MMPs, which are upregulated in aging and photodamaged skin.
Scientific literature suggests that the dipeptide may indirectly influence epigenetic regulation of extracellular matrix turnover, stabilizing mRNA expression of collagen-related enzymes and promoting an optimal cellular microenvironment for matrix deposition. Additionally, palmitoyl dipeptide-7 demonstrates the ability to enhance fibroblast contractility, a property closely related to tissue resilience and wrinkle smoothing.
The compound also contributes to the restoration of the skin barrier function by strengthening intercellular lipid organization and reducing transepidermal water loss (TEWL). Through its lipidated backbone, the peptide reinforces the cohesion of corneocytes and improves the skin’s mechanical integrity, a critical factor in maintaining firmness.
In oxidative stress research, palmitoyl dipeptide-7 exhibits antioxidant behavior by modulating intracellular ROS levels and supporting endogenous antioxidant enzyme systems such as superoxide dismutase (SOD) and catalase. This antioxidative mechanism protects fibroblast mitochondria from damage, prolonging cellular vitality and delaying senescence-associated morphological changes.
Furthermore, palmitoyl dipeptide-7 is thought to impact neurogenic and inflammatory signaling in sensitive skin models. By regulating the release of neuropeptides like substance P and calcitonin gene-related peptide (CGRP), it may help attenuate the inflammatory cascade that contributes to redness and barrier impairment. This multi-modal action profile positions the peptide as a valuable research tool for exploring integrated anti-aging and soothing pathways.
Side Effects
In research use, palmitoyl dipeptide-7 has demonstrated an excellent biocompatibility profile with no cytotoxic or irritant responses at standard experimental concentrations. In vitro assays on human dermal fibroblasts and keratinocytes have shown that it does not trigger pro-inflammatory cytokine release, making it a well-tolerated compound for cellular models of aging and sensitivity.
Potential side effects in non-clinical studies are minimal and typically associated with excessive concentration or improper formulation conditions. Like most peptide compounds, degradation or oxidation may occur if the product is not stored properly; therefore, adherence to controlled storage (2–8 °C) and reconstitution guidelines is crucial to maintain bioactivity.
As this compound is intended strictly for laboratory research use, it should not be applied directly to human or animal skin. Safety assessments in vitro confirm stability and compatibility within cosmetic matrices, but further testing is required for formulation-specific studies. Researchers should use standard biosafety and handling precautions when preparing or analyzing samples.
Keywords
Palmitoyl dipeptide-7, cosmetic peptide, collagen synthesis peptide, anti-aging peptide, skin firmness peptide, extracellular matrix regeneration, fibroblast activation, high-purity peptide, cosmetic research material, peptide supplier China, peptide manufacturer, peptide bulk powder, peptide OEM, peptide wholesale China.
Shipping Guarantee
All shipments are handled using validated cold-chain logistics to preserve peptide integrity. Each package is sealed in moisture-proof containers with secondary protective wrapping and continuous temperature monitoring. Products are shipped via express international couriers with full tracking and insurance coverage.

Trade Assurance
We ensure product authenticity, verified ≥99% purity, and compliance with analytical standards (HPLC, MS, and NMR). Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
All products listed are intended for laboratory research use only and not for human or veterinary use. They are not drugs, medical devices, or diagnostics and should not be administered to humans or animals. Researchers must handle all materials in accordance with institutional biosafety and chemical safety guidelines. The information provided is for scientific reference only and does not imply therapeutic efficacy, safety, or regulatory approval.
Additional information
| Weight | 1.5 kg |
|---|---|
| Dimensions | 62 × 53 × 62 cm |
What is Palmitoyl dipeptide-7 used for in research?
It is primarily studied for collagen renewal, extracellular matrix reconstruction, and anti-aging peptide mechanism research.
What purity level does this peptide have?
All products are verified at ≥99% purity using analytical methods including HPLC and MS.
Is this peptide available in bulk quantities?
Yes, as a factory peptide supplier, we provide OEM & bulk peptide production to support large-scale research needs.
Can this peptide be used in cosmetic formulation studies?
Yes, it is suitable for in vitro and formulation research focused on peptide delivery and stability.
How should Palmitoyl dipeptide-7 be stored?
Store at 2–8 °C in a sealed, moisture-free environment to preserve peptide integrity.
Is this peptide suitable for sensitive-skin research?
Yes, due to its soothing and matrix-stabilizing effects, it is often included in sensitive-skin model research.
Does your company provide Certificates of Analysis (CoA)?
Yes, each batch is accompanied by a CoA confirming purity, molecular weight, and analytical data.
Can I order directly from your China peptide factory?
Yes, we are a verified China peptide manufacturer offering direct wholesale and OEM services.
What delivery methods are available for international shipping?
We use validated cold-chain express couriers with full insurance and tracking.
Are high-purity cosmetic peptides available at low prices?
We are known as a low-price peptide supplier offering high-purity cosmetic peptides directly from the factory.
Is Palmitoyl dipeptide-7 synthetic or natural?
It is a synthetic biomimetic peptide designed to replicate natural skin-regenerative signals.
What is your minimum order quantity (MOQ)?
MOQ can vary based on project scale; we support both laboratory-scale and bulk orders.
Do you support OEM packaging for cosmetic raw material manufacturers?
Yes, OEM & private label packaging is available for international cosmetic raw material manufacturers.
What analytical tests are performed on this peptide?
Each batch undergoes HPLC, MS, and NMR confirmation to ensure identity, purity, and consistency.
How can I request a quotation for wholesale peptide supply?
You can contact our sales department for peptide wholesale China pricing and high-purity supply options.












Reviews
There are no reviews yet.