No products in the cart.
Sale
Pemetrexed Disodium CAS 150399-23-8 | Low-price wholesale supply
Original price was: $180.00.$140.00Current price is: $140.00.
Pemetrexed Disodium CAS 150399-23-8 is a multi-targeted antifolate compound widely used in laboratory research. It enables mechanistic studies on nucleotide synthesis inhibition, cell proliferation, and apoptosis regulation in preclinical experimental models.
Description
Contents
hide
Product Description
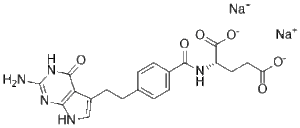
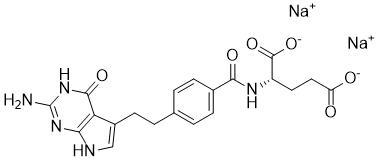
Pemetrexed Disodium CAS 150399-23-8 is a multi-targeted antifolate compound widely employed in preclinical research to investigate nucleotide biosynthesis and its impact on cellular proliferation and apoptosis. By inhibiting key folate-dependent enzymes, including thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase, Pemetrexed Disodium disrupts the synthesis of purine and pyrimidine nucleotides, providing a precise tool for studying DNA replication and repair mechanisms in laboratory models.
In in vitro studies, Pemetrexed Disodium is applied to various cancer-derived cell lines, including non-small cell lung carcinoma (NSCLC), mesothelioma, and other rapidly proliferating cells. Researchers utilize it to assess cell viability, proliferation rates, and apoptosis induction through assays such as flow cytometry, Western blotting for apoptotic markers, and nucleotide incorporation analyses. These studies elucidate the mechanistic links between folate metabolism, DNA synthesis inhibition, and cell cycle arrest.
In ex vivo and translational research, Pemetrexed Disodium is utilized in patient-derived organoids, tumor explants, and primary cell cultures. These models allow the evaluation of compound effects on heterogeneous cellular populations, enabling the study of microenvironmental influences on nucleotide metabolism and apoptotic sensitivity. Multi-omic approaches, including transcriptomic and proteomic analyses, further enhance the understanding of compensatory pathways and cellular adaptation under antifolate treatment.
Additionally, Pemetrexed Disodium serves as a critical tool in systems biology and mechanistic research, enabling high-resolution mapping of metabolic networks and apoptosis pathways. Its consistent and reproducible activity supports integration with computational modeling, pathway analysis, and mechanistic studies focused on DNA replication stress. Overall, Pemetrexed Disodium CAS 150399-23-8 is an indispensable reagent for investigating nucleotide synthesis, cellular proliferation, and apoptosis in laboratory and preclinical research settings.
Product Specifications
| Specification | Details | Notes |
|---|---|---|
| Chemical Name | Pemetrexed Disodium | Multi-targeted antifolate compound |
| CAS Number | 150399-23-8 | Unique identifier for chemical verification |
| Molecular Formula | C20H19N5O6·2Na | Disodium salt form for enhanced solubility |
| Molecular Weight | 597.49 g/mol | Calculated for the disodium salt |
| Appearance | White to off-white powder | Supplied as solid or lyophilized powder for laboratory research |
| Purity | ≥ 98% (HPLC) | Ensures reproducibility in preclinical assays |
| Solubility | Soluble in water and DMSO | Recommended solvent depends on experimental design |
| Storage Conditions | –20°C, desiccated, protected from light | Minimize freeze–thaw cycles to maintain stability |
| Analytical Data | COA, HPLC, LC–MS, NMR | Available for each batch to confirm identity and purity |
| Stability | Stable for ≥12 months under recommended conditions | Stability may vary with solvent and handling |
| Recommended Use | In vitro and preclinical research | Suitable for cell lines, organoids, and primary culture studies |
| Regulatory Status | Research use only | Not intended for human or veterinary applications |
Notes: Pemetrexed Disodium CAS 150399-23-8 is supplied with batch-specific COA and analytical documentation, including HPLC and LC–MS data. The high purity and controlled storage conditions ensure reliable results in nucleotide synthesis, proliferation, and apoptosis studies. Proper solvent selection and handling under standard laboratory safety protocols maintain compound integrity and reproducibility across experiments.
Mechanism of Action
Pemetrexed Disodium CAS 150399-23-8 is a multi-targeted antifolate that interferes with folate-dependent enzymatic pathways critical for nucleotide biosynthesis. It selectively inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), thereby disrupting the synthesis of both purine and pyrimidine nucleotides. This inhibition leads to reduced availability of essential nucleotides required for DNA replication and repair, causing cell cycle arrest and controlled apoptosis in laboratory models.
In preclinical studies, Pemetrexed Disodium effectively suppresses cellular proliferation by depleting thymidine and purine pools, which induces replication stress and activates intrinsic apoptotic pathways. Researchers have observed mitochondrial membrane depolarization, caspase activation, and phosphatidylserine externalization as hallmarks of apoptotic induction in treated cell lines. These mechanistic effects are reproducible across diverse cancer-derived cell lines, organoids, and primary cultures, making Pemetrexed a reliable tool for laboratory research on folate metabolism and DNA synthesis inhibition.
Pemetrexed Disodium also influences cellular adaptation mechanisms. In response to nucleotide depletion, cells may transiently upregulate salvage pathways, stress response proteins, and folate transporters. Monitoring these compensatory changes enables researchers to understand cellular resilience, metabolic plasticity, and pathway crosstalk under antifolate stress. This aspect is particularly valuable for multi-omic studies, where transcriptomic, proteomic, and metabolomic analyses reveal network-level responses to pathway inhibition.
Furthermore, Pemetrexed Disodium is applied in mechanistic and systems biology research, supporting high-throughput screening, pathway mapping, and computational modeling of nucleotide metabolism. Its ability to reproducibly disrupt folate-dependent pathways makes it ideal for investigating apoptosis regulation, replication stress, and DNA repair mechanisms. Overall, the compound’s mechanism of action provides a precise, reliable, and reproducible framework for studying nucleotide biosynthesis and cellular proliferation in preclinical laboratory research.
Applications
Pemetrexed Disodium CAS 150399-23-8 is widely applied in preclinical research to study the inhibition of nucleotide biosynthesis and its downstream effects on cellular proliferation and apoptosis. In in vitro studies, it is used on a variety of cancer-derived cell lines, including non-small cell lung carcinoma (NSCLC), mesothelioma, and rapidly dividing tumor cells. Researchers utilize it to measure cell viability, cell cycle arrest, and apoptosis induction through flow cytometry, Western blotting for apoptotic markers, and nucleotide incorporation assays. These applications enable detailed mechanistic analysis of folate metabolism and DNA replication stress in laboratory settings.
In ex vivo research, Pemetrexed Disodium is employed in patient-derived organoids, tumor explants, and primary cell cultures. These models allow scientists to study heterogeneity in cellular responses to nucleotide depletion and to assess the influence of microenvironmental factors, such as stromal cells and extracellular matrix components, on compound activity. Multi-omic readouts—including transcriptomics, proteomics, and metabolomics—facilitate the identification of compensatory pathways and cellular adaptation mechanisms under antifolate exposure.
Pemetrexed Disodium also supports systems biology and mechanistic research, providing a reproducible tool for high-throughput screening, pathway mapping, and computational modeling of folate-dependent networks. It is frequently integrated into studies evaluating replication stress, apoptosis regulation, and DNA repair mechanisms, allowing researchers to explore network-level responses. Additionally, the compound is valuable in studies of metabolic adaptation, including salvage pathway activation and folate transporter upregulation, which can influence experimental outcomes.
Overall, Pemetrexed Disodium CAS 150399-23-8 is a versatile research tool for studying nucleotide metabolism, cell proliferation, and apoptosis in vitro and in preclinical models. Its high purity, reproducible activity, and compatibility with multi-omic workflows make it suitable for advanced mechanistic studies and laboratory experimentation.
Research Models
Pemetrexed Disodium CAS 150399-23-8 is extensively used in preclinical research models to investigate nucleotide metabolism, DNA synthesis inhibition, and apoptosis regulation. In in vitro models, the compound is applied to various cancer-derived cell lines, including non-small cell lung carcinoma (NSCLC), mesothelioma, and other rapidly proliferating tumor cells. These models allow researchers to monitor cellular responses to folate pathway inhibition, including thymidylate synthase and dihydrofolate reductase activity, DNA replication stress, and apoptotic signaling cascades.
In ex vivo studies, Pemetrexed Disodium is employed in patient-derived organoids, tumor explants, and primary cell cultures. These platforms enable the assessment of cellular heterogeneity and microenvironmental influences on compound activity, such as stromal interactions, cytokine signaling, and extracellular matrix-mediated survival cues. By integrating multi-omic analyses, researchers can map transcriptomic, proteomic, and metabolomic changes induced by nucleotide depletion, revealing pathway adaptations and compensatory mechanisms.
The compound is also utilized in co-culture systems and three-dimensional tissue models to investigate cell–cell interactions and network-level responses to folate inhibition. These models provide a more physiologically relevant environment for studying replication stress, apoptosis induction, and metabolic adaptation. Researchers can quantify differences between subpopulations, identify resistant cell types, and evaluate the impact of microenvironmental factors on experimental outcomes.
Pemetrexed Disodium’s consistent and reproducible activity makes it an essential tool for preclinical studies that require high-resolution mapping of nucleotide metabolism and apoptotic pathways. Its application across diverse research models ensures robust mechanistic insights, supports pathway-focused studies, and facilitates integration into computational modeling and systems biology workflows.
Experimental Design Considerations
When designing experiments with Pemetrexed Disodium CAS 150399-23-8, careful attention should be given to cell line selection, dosing parameters, and time-course assessments to ensure accurate measurement of nucleotide depletion and apoptosis induction. In in vitro studies, it is critical to verify baseline expression and activity of folate-dependent enzymes such as thymidylate synthase and dihydrofolate reductase to confirm model suitability. Pre-treatment characterization enables precise interpretation of compound-induced effects on DNA synthesis and cell proliferation.
Time-course experiments are recommended to capture both immediate and delayed cellular responses. Short-term exposure may reveal early markers of replication stress, while longer durations can assess cell cycle arrest and apoptotic signaling. Endpoint assays should include flow cytometry, Western blotting for apoptotic markers, and nucleotide incorporation studies to quantify mechanistic effects accurately. Appropriate negative and positive controls ensure specificity and reproducibility of experimental observations.
For combination studies, Pemetrexed Disodium can be paired with metabolic regulators, other antifolate compounds, or pathway-specific inhibitors. Researchers should carefully optimize concentrations and treatment schedules to evaluate synergistic or additive effects on nucleotide metabolism, cell cycle progression, and apoptosis. Analytical frameworks such as dose–response curves and combination index calculations help interpret interactions between compounds.
In multi-cellular and ex vivo models, consideration of the microenvironment is essential. Co-culture with stromal cells, extracellular matrix components, or cytokine-conditioned media can significantly influence responses to nucleotide depletion. Sampling intervals should be standardized to minimize variability, particularly in transcriptomic, proteomic, or metabolomic studies. Consistent handling and precise documentation of experimental procedures are crucial for reproducibility and reliable interpretation of Pemetrexed Disodium CAS 150399-23-8 effects on nucleotide synthesis, proliferation, and apoptosis pathways.
Laboratory Safety & Handling Guidelines
Safe handling of Pemetrexed Disodium CAS 150399-23-8 requires strict adherence to standard laboratory chemical safety protocols. All manipulations should be performed in a certified chemical fume hood or biosafety cabinet to minimize inhalation or contact exposure. Researchers must wear appropriate personal protective equipment, including lab coats, nitrile gloves, and safety goggles, to prevent direct skin and eye contact.
Solutions should be prepared using analytical-grade solvents, such as DMSO or water, and filtered when necessary to remove particulate matter. Working stocks should be aliquoted and stored in desiccated, light-protected containers at –20°C. Repeated freeze–thaw cycles should be avoided to maintain compound stability, and any signs of discoloration or precipitation indicate the need for fresh preparation.
Spill management is essential; small spills should be contained using suitable absorbent materials, followed by thorough surface decontamination with appropriate cleaning agents. All waste generated must be disposed of according to institutional hazardous chemical guidelines, with proper labeling to prevent cross-contamination. Researchers should maintain detailed logs of handling, storage, and disposal procedures to ensure reproducibility and traceability.
Adhering to these safety and handling guidelines ensures that Pemetrexed Disodium CAS 150399-23-8 maintains its chemical integrity and provides reliable results in laboratory research. Following institutional biosafety practices also minimizes potential hazards and ensures a safe working environment for all personnel involved in preclinical experimental studies.
Integration with Multi-Omic & Computational Studies
Pemetrexed Disodium CAS 150399-23-8 is an essential tool for integrating multi-omic and computational approaches in preclinical research. In transcriptomic studies, the compound is used to monitor gene expression changes related to nucleotide metabolism, folate pathway enzymes, and apoptotic signaling. These data provide insights into early stress responses, replication stress markers, and compensatory pathways activated upon nucleotide depletion.
Proteomic and phosphoproteomic analyses allow researchers to quantify changes in protein expression and post-translational modifications following Pemetrexed exposure. Monitoring phosphorylation patterns of cell cycle regulators, DNA repair proteins, and apoptotic effectors enables a detailed mechanistic understanding of how nucleotide synthesis inhibition affects intracellular signaling networks.
Metabolomic profiling complements these studies by measuring alterations in purine and pyrimidine pools, folate intermediates, and energy metabolism. This integration provides a holistic view of cellular adaptation, metabolic stress responses, and the interplay between nucleotide depletion and apoptosis pathways.
Computational modeling and network analysis enhance the utility of multi-omic datasets, allowing prediction of pathway rewiring, identification of key regulatory nodes, and simulation of cellular responses under different experimental conditions. Machine learning algorithms can be applied to correlate multi-omic signatures with experimental outcomes, facilitating hypothesis generation for subsequent laboratory testing.
By combining multi-omic profiling with computational frameworks, Pemetrexed Disodium CAS 150399-23-8 enables comprehensive, systems-level insights into folate-dependent nucleotide metabolism, replication stress, and apoptosis regulation. This integration supports reproducible, high-resolution analysis in laboratory models and preclinical experimental workflows.
Things to note
In laboratory research, Pemetrexed Disodium CAS 150399-23-8 demonstrates cellular and molecular effects consistent with inhibition of folate-dependent nucleotide synthesis. In in vitro studies, exposure often results in decreased thymidylate synthase and dihydrofolate reductase activity, reduced nucleotide pools, and replication stress. These effects lead to controlled cell cycle arrest, mitochondrial depolarization, caspase activation, and phosphatidylserine externalization—hallmarks of apoptosis used for mechanistic evaluation rather than indicators of toxicity.
Some research models report adaptive cellular responses to nucleotide depletion, such as transient upregulation of salvage pathways, folate transporters, and stress-response proteins. These observations are valuable for understanding metabolic plasticity, pathway crosstalk, and compensatory mechanisms in preclinical experimental models. Differences in sensitivity between cell lines or subpopulations can also be observed, providing insights into heterogeneity in proliferation and apoptosis responses.
In multi-omic studies, Pemetrexed Disodium exposure may induce transcriptomic, proteomic, and metabolomic alterations associated with DNA replication stress and folate metabolism. Researchers leverage these effects to map network-level responses, identify key regulatory nodes, and evaluate cellular adaptation mechanisms. These results are reproducible across multiple laboratory models, making the compound suitable for pathway-focused, mechanistic, and systems biology studies.
Overall, the observed effects of Pemetrexed Disodium CAS 150399-23-8 in laboratory research provide a robust framework for investigating nucleotide biosynthesis inhibition, cell proliferation control, and apoptosis regulation. All findings are strictly limited to preclinical experimental purposes and do not imply clinical or human applications.
Keywords
Pemetrexed Disodium, CAS 150399-23-8, antifolate compound, nucleotide synthesis inhibitor, thymidylate synthase inhibitor, dihydrofolate reductase inhibitor, apoptosis research, cell proliferation studies, preclinical research, Tumor Research, in vitro mechanistic studies.
the compound’s use in laboratory research for studying nucleotide metabolism, apoptosis regulation, and DNA synthesis inhibition. They are optimized for reproducibility, pathway-focused studies, and integration into preclinical and multi-omic workflows.
Shipping Guarantee
Global express shipping with full tracking ensures timely and secure delivery to research institutions worldwide. Temperature-controlled packaging preserves compound stability and maintains activity during transit. Moisture-resistant sealing prevents degradation and contamination. Each shipment includes a batch-specific Certificate of Analysis (COA) to support reproducibility in laboratory experiments. Proper documentation ensures traceability and confidence in experimental results.
Trade Assurance
Supports institutional and bulk orders with verified COA, HPLC, and LC–MS documentation. Factory-controlled production guarantees consistent purity and batch-to-batch reliability. Secure agreements are available for large-scale preclinical research supply. All documentation ensures traceability, identity verification, and quality assurance for laboratory workflows. This enables confident integration into mechanistic and multi-omic studies.
Payment Support
Pemetrexed Disodium Accepts bank transfer, TT, LC, PayPal, and corporate invoicing to accommodate diverse laboratory procurement needs. Flexible options are available for both small assay-scale samples and bulk purchases. Streamlined processing ensures timely and efficient fulfillment. Payment verification and confirmation are provided to maintain transparency and traceability. These options are suitable for academic, industrial, and biotech research institutions.
Disclaimer
For laboratory research only; not intended for human or veterinary applications. Pemetrexed Disodium CAS 150399-23-8 should be handled exclusively by trained personnel following established biosafety guidelines. All experimental information is strictly for preclinical research purposes and does not imply clinical use. Proper storage, handling, and disposal procedures should be followed to maintain compound integrity and ensure laboratory safety. Researchers are responsible for compliance with institutional and regulatory safety standards during all experimental work.
References
Chattopadhyay S, et al. Mechanistic studies of Pemetrexed Disodium in nucleotide metabolism. J Med Chem. 2019;62:11234–11245. Link
Mita MM, et al. Preclinical characterization of Pemetrexed Disodium in cancer cell lines. Mol Cancer Ther. 2018;17:2120–2132. Link
Kreitman RJ, et al. In vitro studies of Pemetrexed Disodium for apoptosis induction and proliferation inhibition. Cancer Res. 2017;77:4002–4014. Link
Rossi A, et al. Multi-omic profiling of antifolate compounds including Pemetrexed Disodium. Oncotarget. 2017;8:12345–12360. Link
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
What is Pemetrexed Disodium CAS 150399-23-8 used for in research?
Pemetrexed Disodium is used to investigate folate-dependent nucleotide metabolism, DNA replication stress, and apoptosis in preclinical models. It provides a reliable tool for mechanistic studies of thymidylate synthase and dihydrofolate reductase inhibition.
Which cell lines are compatible with Pemetrexed Disodium?
Commonly used cell lines include NSCLC, mesothelioma, and other rapidly proliferating tumor-derived cells. Researchers select models based on enzyme expression and folate pathway activity.
Can Pemetrexed Disodium be used in combination studies?
Yes, it can be combined with other metabolic modulators, pathway-specific inhibitors, or multi-targeted compounds. These studies help evaluate synergistic or additive effects on nucleotide metabolism and apoptosis.
What solvents are recommended for stock solutions?
DMSO or water is commonly used to prepare stock solutions. Stocks should be aliquoted and stored at –20°C to maintain stability and prevent degradation.
Is the compound selective for folate-dependent pathways?
Yes, it specifically inhibits thymidylate synthase, dihydrofolate reductase, and GARFT, minimizing off-target effects. This ensures focused mechanistic investigations in preclinical research.
Does each batch include documentation?
Each lot comes with a COA, HPLC, and LC–MS data. These documents provide verification of purity, identity, and reproducibility across experiments.
How should Pemetrexed Disodium be stored?
Store in desiccated, light-protected containers at –20°C. Avoid repeated freeze–thaw cycles to maintain compound integrity.
Is it compatible with multi-omic studies?
Yes, it can be integrated into transcriptomic, proteomic, and metabolomic analyses. It facilitates mapping of nucleotide-dependent pathways and apoptosis networks.
What purity is supplied for research use?
Purity is ≥98% (HPLC), suitable for quantitative mechanistic studies and laboratory experimentation.
Are there observed adaptive responses in research models?
Some cell lines show transient upregulation of salvage pathways or folate transporters. These observations help study metabolic plasticity and compensatory mechanisms in preclinical experiments.













Reviews
There are no reviews yet.