No products in the cart.
Pirtobrutinib 100mg | High purity | Factory manufactured
$2.00
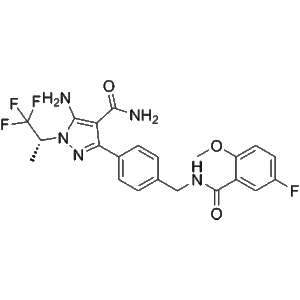
Pirtobrutinib is a high-purity small-molecule research compound, factory manufactured and supplied at low-price wholesale scale, specifically developed for in vitro biochemical assays, molecular interaction analysis, and signaling pathway investigations. This product is intended exclusively for laboratory research use.
Description
Product Description
Pirtobrutinib is a high-purity, factory-manufactured small-molecule research compound designed specifically for in vitro laboratory investigations, molecular mechanism exploration, and signal transduction pathway studies. Supplied as part of a low-price wholesale research material portfolio, this compound supports reproducible, scalable experimental workflows across academic, industrial, and contract research environments.
From a chemical research standpoint, Pirtobrutinib is valued for its well-characterized molecular structure and predictable interaction behavior within controlled experimental systems. Its non-covalent binding profile enables researchers to explore dynamic molecular interactions, reversible binding kinetics, and structure–function relationships without permanent target modification, making it particularly suitable for comparative mechanistic analysis and pathway interrogation.
In laboratory research settings, Pirtobrutinib is frequently employed as a reference compound in kinase-focused biochemical assays, where precise modulation of enzymatic activity is required to dissect downstream signaling events. Its consistent purity profile and controlled manufacturing process ensure batch-to-batch reproducibility, a critical factor for longitudinal studies, parallel screening experiments, and multi-site collaborative research projects.
The compound’s physicochemical stability allows seamless integration into cell-free enzymatic systems, recombinant protein assays, and pathway reconstruction models, supporting high-resolution investigation of molecular binding behavior under variable experimental conditions. Researchers utilize Pirtobrutinib to evaluate binding affinity trends, competitive interaction profiles, and conformational adaptability, often in combination with analytical techniques such as HPLC-based monitoring, spectroscopic evaluation, or computational docking validation.
As a factory-manufactured research reagent, this Pirtobrutinib product is produced under standardized quality control protocols, ensuring traceability, analytical transparency, and scalability from gram-level quantities to bulk wholesale supply. This makes it particularly suitable for screening campaigns, SAR development pipelines, and method development studies, where consistent material supply is essential.
Importantly, this product is positioned exclusively for laboratory research use. All descriptions, specifications, and performance characteristics relate solely to in vitro experimental models and molecular-level investigations. No implications are made regarding administration, physiological exposure, or any non-research application. The compound serves purely as a chemical tool for mechanistic insight, pathway mapping, and experimental validation within controlled laboratory environments.

Product Specifications
| Parameter | Specification |
|---|---|
| Product Name | Pirtobrutinib |
| Chemical Type | Synthetic small-molecule research compound |
| Purity | ≥99.0% (HPLC verified) |
| Analytical Methods | HPLC, NMR, MS (batch-dependent documentation) |
| Appearance | White to off-white crystalline solid |
| Molecular Weight | Refer to batch-specific analytical documentation |
| Molecular Formula | Refer to certificate of analysis (COA) |
| Solubility (Research Evaluation) | Soluble in DMSO and selected laboratory-grade organic solvents |
| Stability Profile | Chemically stable under recommended laboratory storage conditions |
| Hygroscopicity | Low to moderate (evaluate during experimental preparation) |
| Manufacturing Process | Factory manufactured under standardized quality control |
| Batch Consistency | High batch-to-batch reproducibility |
| Packaging Options | Standard vials, bulk containers, custom packaging available |
| Supply Scale | Gram-level to multi-kilogram wholesale supply |
| Intended Use | Laboratory research only (in vitro applications) |
| Custom Services | Bulk supply, purity customization, documentation support |
Notes
Pirtobrutinib supplied under this specification is produced through a controlled chemical synthesis workflow, followed by multi-stage purification to ensure high structural integrity and analytical consistency. Each production batch undergoes internal quality verification using HPLC purity profiling, with supplementary structural confirmation methods such as NMR and mass spectrometry applied as required for research-grade documentation.
The compound’s high purity (≥99.0%) minimizes background interference in sensitive biochemical and molecular assays, making it particularly suitable for enzyme interaction studies, signal pathway reconstruction, and structure–activity relationship (SAR) investigations. Researchers benefit from predictable experimental behavior when comparing results across different assay platforms or study timelines.
From a supply-chain perspective, this product is factory manufactured and scalable, enabling reliable long-term procurement for laboratories conducting high-throughput screening, method development, or multi-phase research programs. Low-price wholesale supply options support cost-efficient experimental expansion without compromising material quality.
Solubility characteristics are optimized for laboratory preparation and in vitro assay compatibility, allowing flexible integration into diverse experimental designs. Researchers are advised to evaluate solvent systems and concentration ranges appropriate to their specific assay conditions to maintain data accuracy and reproducibility.
All specifications provided here relate strictly to research material characteristics and laboratory handling considerations. Pirtobrutinib is supplied solely as a chemical research tool, intended to support molecular-level investigations under controlled experimental environments.
Mechanism of Action
Molecular Binding Characteristics
Pirtobrutinib functions as a reversible small-molecule interaction probe within controlled laboratory research systems. In in vitro experimental models, the compound is utilized to examine non-covalent binding behavior at kinase-associated domains, enabling detailed evaluation of binding orientation, affinity modulation, and dissociation dynamics. Unlike irreversible interaction models, this reversible binding profile allows researchers to observe dynamic equilibrium states, making Pirtobrutinib particularly valuable for time-resolved molecular studies.
Signal Pathway Modulation in Research Models
Within reconstructed signaling pathway systems, Pirtobrutinib is applied as a selective pathway-modulating tool to investigate how targeted molecular interactions influence downstream signal propagation and feedback regulation. By introducing controlled levels of pathway modulation in vitro, researchers can dissect signal amplification, attenuation, and redundancy mechanisms without permanently altering pathway components. This controlled modulation supports comparative studies across parallel experimental conditions.
Structure–Function Relationship Analysis
The chemical architecture of Pirtobrutinib supports structure–function relationship (SFR) investigations, where subtle variations in molecular conformation are correlated with measurable changes in interaction efficiency and pathway response. In silico modeling and molecular docking simulations are often paired with experimental assays to validate binding-site accessibility and conformational flexibility, providing complementary insights into molecular behavior.
In advanced laboratory research designs, Pirtobrutinib is incorporated into mechanism-focused studies exploring altered binding environments. By evaluating interaction performance under modified experimental parameters, researchers gain insight into adaptive molecular responses and alternative interaction modes. These studies contribute to a deeper understanding of kinase-domain adaptability and interaction resilience at the molecular level.
Applications
In Vitro Biochemical Assays
Pirtobrutinib is widely applied in in vitro biochemical assay systems designed to investigate molecular interaction behavior and enzyme-associated signal modulation. Within cell-free experimental environments, the compound serves as a precision research tool for evaluating binding affinity, interaction selectivity, and competitive interaction profiles. Its consistent purity and predictable chemical properties allow researchers to generate reproducible datasets across parallel assay conditions, supporting quantitative comparison and assay optimization.
Signal Transduction Pathway Research
In laboratory-constructed signaling pathway models, Pirtobrutinib is utilized to explore pathway connectivity, signal propagation, and feedback regulation mechanisms. By introducing controlled pathway modulation under in vitro conditions, researchers can analyze signal cascade responses and identify key regulatory nodes within complex signaling networks. These applications are particularly valuable for pathway mapping and mechanistic hypothesis validation.
Molecular Interaction & Structural Studies
Pirtobrutinib supports molecular interaction research focused on understanding how small molecules engage with kinase-associated domains at the structural level. The compound is frequently incorporated into binding-site mapping, conformational analysis, and interaction stability studies, often in combination with spectroscopic methods or structure-based computational modeling. These studies contribute to a deeper understanding of structure–interaction relationships.
Screening & Comparative Research Models
In screening-oriented research workflows, Pirtobrutinib functions as a reference or comparator compound within controlled experimental panels. Researchers employ it to benchmark interaction behavior, evaluate assay sensitivity, and validate screening methodologies. Its compatibility with high-throughput screening (HTS) platforms makes it suitable for early-stage exploratory research and comparative molecular profiling.
Computational & Multi-Omic Integration
Pirtobrutinib is also integrated into computational modeling and multi-omic research frameworks, where experimental interaction data are correlated with predictive simulations and molecular datasets. This combined approach enhances mechanistic interpretation and data robustness, supporting comprehensive laboratory-based research strategies.
Research Models
Cell-Free Enzymatic Systems
Pirtobrutinib is extensively utilized in cell-free enzymatic research models to investigate molecular interaction dynamics under tightly controlled experimental conditions. These systems enable precise evaluation of binding behavior, interaction reversibility, and kinetic response patterns without interference from complex biological variables. Researchers commonly employ such models to generate high-resolution data on enzyme-associated signal modulation and interaction specificity, supporting foundational mechanistic studies.
Recombinant Protein Interaction Models
In recombinant protein-based research platforms, Pirtobrutinib serves as a valuable tool for examining direct molecular engagement with isolated protein domains. These models allow researchers to focus on structure-driven interaction mechanisms, facilitating detailed analysis of binding orientation, affinity trends, and conformational adaptability. The high purity and batch consistency of Pirtobrutinib contribute to reproducible experimental outcomes, which are essential for comparative and longitudinal research designs.
In Vitro Pathway Reconstruction Models
Pirtobrutinib is also integrated into in vitro reconstructed signaling pathway models, where individual pathway components are assembled to simulate defined signaling events. Within these models, researchers can assess pathway modulation effects, feedback mechanisms, and signal propagation patterns in a stepwise and interpretable manner. Such models are particularly useful for dissecting complex signaling networks into experimentally manageable units.
Screening-Oriented Research Frameworks
In screening-based research models, Pirtobrutinib is applied as a benchmark or reference compound to validate assay performance and evaluate experimental sensitivity. Its predictable interaction profile makes it suitable for comparative screening panels and methodological optimization, including early-stage exploratory studies and assay development workflows.
Computationally Integrated Research Models
Pirtobrutinib is frequently incorporated into hybrid research models that combine experimental data with computational simulations and predictive modeling. By correlating in vitro interaction results with in silico analyses, researchers gain deeper insight into molecular behavior, binding stability, and mechanistic plausibility, strengthening overall research conclusions.
Experimental Design Considerations
When incorporating Pirtobrutinib into laboratory-based research workflows, careful experimental design is essential to ensure data reliability, interpretability, and reproducibility. As a small-molecule research compound, its performance in vitro can be influenced by multiple controllable variables that should be systematically evaluated during assay development and optimization.
One key consideration is compound preparation and solvent compatibility. Pirtobrutinib is commonly evaluated in controlled solvent systems suitable for biochemical and molecular assays. Researchers should confirm solvent tolerance thresholds within their specific experimental setup to minimize background effects and ensure accurate interpretation of interaction behavior. Gradual concentration titration is recommended during preliminary studies to establish appropriate working ranges for downstream analysis.
Temporal parameters also play a critical role in experimental outcomes. Time-dependent interaction studies allow researchers to observe binding dynamics, equilibrium behavior, and dissociation patterns under defined conditions. Incorporating multiple incubation time points can provide insight into molecular stability and interaction reversibility within the assay system.
The inclusion of appropriate controls and reference conditions is another essential design element. Parallel negative and baseline controls help distinguish compound-specific effects from system variability, while reference compounds or internal standards support comparative analysis. Replicate measurements and randomized sample handling further enhance statistical robustness.
Environmental conditions such as temperature, buffer composition, and pH stability should be standardized across experiments to reduce variability. Minor fluctuations in these parameters may influence molecular interaction profiles, particularly in sensitive enzymatic or binding assays.
Finally, data interpretation should be supported by orthogonal validation approaches, such as combining biochemical readouts with structural or computational analyses. Integrating multiple analytical perspectives strengthens mechanistic conclusions and supports confident experimental decision-making within laboratory research frameworks.
Laboratory Safety & Handling Guidelines
Pirtobrutinib is supplied as a high-purity laboratory research compound and should be handled in accordance with standard chemical safety practices applicable to small-molecule reagents used in in vitro and molecular mechanism research. All handling procedures should be conducted by trained laboratory personnel within controlled research environments.
Personal protective equipment (PPE) is recommended during all stages of handling and preparation. This typically includes laboratory gloves, protective eyewear, and appropriate lab coats to minimize the risk of accidental contact. Direct contact with skin, eyes, or clothing should be avoided, and all manipulations should be performed using suitable laboratory tools rather than direct handling.
Work involving Pirtobrutinib should be carried out in well-ventilated laboratory spaces or designated chemical handling areas. When preparing solutions or transferring material, researchers should take precautions to reduce the generation of airborne particulates. If fine material handling is required, the use of localized containment or fume-controlled workstations is advised to maintain a clean and controlled environment.
Storage conditions play an important role in maintaining compound integrity. Pirtobrutinib should be stored in tightly sealed containers, clearly labeled, and kept under conditions recommended by internal laboratory protocols. Separation from incompatible chemicals and protection from excessive moisture or environmental exposure help preserve material stability during long-term research use.
In the event of a minor spill, standard laboratory cleanup procedures should be followed. Solid material should be collected using appropriate tools and disposed of according to institutional chemical waste guidelines. Contaminated surfaces should be cleaned using compatible solvents and absorbent materials approved for laboratory use.
Waste disposal must comply with local regulations and institutional policies governing chemical research materials. Pirtobrutinib and any solutions prepared from it should be treated as research chemical waste and handled through authorized disposal channels.
These guidelines are intended to support safe, responsible laboratory research practices and ensure consistent handling throughout experimental workflows.
Integration with Multi-Omic & Computational Studies
Multi-Omic Data Integration in Laboratory Research
Pirtobrutinib is frequently incorporated into multi-omic research frameworks to support comprehensive molecular-level investigations under controlled in vitro conditions. Within proteomic workflows, the compound is applied to evaluate protein interaction patterns, pathway-associated expression shifts, and network connectivity changes resulting from targeted molecular modulation. These datasets enable researchers to contextualize biochemical assay results within broader molecular landscapes.
In transcriptomic research models, Pirtobrutinib-related experimental outputs are analyzed alongside gene expression profiles to explore correlations between molecular interaction events and downstream regulatory responses. Although all observations remain strictly within laboratory research contexts, this integrative approach supports hypothesis generation and pathway mapping across multiple data layers.
Metabolomic datasets may also be used in parallel experimental designs to assess pathway-associated molecular flux and network balance, providing an additional layer of mechanistic insight when combined with interaction-focused studies.
Computational Modeling & In Silico Analysis
Pirtobrutinib plays a key role in computational research workflows, where experimental data are paired with molecular docking, binding-site prediction, and structural simulation tools. In silico modeling enables researchers to visualize potential interaction conformations, assess binding stability, and explore theoretical interaction scenarios prior to or alongside laboratory experimentation.
Computational approaches also support structure–activity relationship (SAR) modeling, allowing researchers to correlate experimental interaction data with predicted molecular features. These analyses enhance experimental efficiency by guiding assay design and refining mechanistic interpretation.
Data-Driven Mechanistic Validation
By integrating multi-omic datasets with computational predictions, researchers can perform cross-validation of observed molecular behaviors, strengthening confidence in experimental conclusions. This data-driven strategy supports robust mechanistic exploration, promotes reproducibility, and facilitates deeper understanding of complex signaling systems within laboratory research environments.
Things to Note
Pirtobrutinib is strictly for laboratory research use and should not be interpreted or applied for any clinical, in vivo, or therapeutic purposes.
All handling and experimental procedures should follow institutional chemical safety protocols to ensure researcher safety and maintain compound integrity.
Prior to use, researchers should verify solvent compatibility, concentration ranges, and assay conditions to optimize experimental accuracy and reproducibility.
Maintain proper storage conditions, including tightly sealed containers, controlled temperature, and protection from moisture or light, to preserve compound stability.
During solution preparation or manipulation, minimize exposure to dust or airborne particulates and use appropriate laboratory tools to reduce contamination risk.
Incorporate proper experimental controls and replicates to validate molecular interaction outcomes and support data reliability.
Waste materials and any unused compound should be disposed of according to institutional chemical waste regulations, ensuring environmental safety.
Researchers are advised to consult batch-specific analytical documentation such as COA, HPLC profiles, and structural verification prior to experimental use.
Pirtobrutinib’s behavior may vary with assay type, solvent system, and incubation parameters; careful preliminary evaluation is recommended for optimal experimental design.
Ensure that all personnel handling the compound are adequately trained in laboratory safety and are aware of compound-specific handling requirements.
Keywords
Pirtobrutinib, high purity Pirtobrutinib, Pirtobrutinib research compound, Pirtobrutinib factory manufactured, Pirtobrutinib wholesale supply, Pirtobrutinib in vitro, Pirtobrutinib molecular mechanism, Pirtobrutinib signaling pathway,Tumor (compound) Research, Pirtobrutinib laboratory research
Shipping Guarantee
Secure laboratory-grade packaging
Temperature-appropriate logistics
Global shipping support for research institutions
Trade Assurance
Factory-direct supply with documented quality control, batch traceability, and contractual trade assurance for bulk and wholesale orders.
Payment Support
We support multiple international payment methods, including:
Credit Card
T/T (Telegraphic Transfer)
Cryptocurrency (encrypted payment support available)
Disclaimer
For laboratory research use only.
Not intended for administration, diagnostic, or any non-research application. No claims are made regarding physiological, clinical, or biological outcomes beyond controlled in vitro research settings.
References
PubChem Compound Database – Pirtobrutinib
https://pubchem.ncbi.nlm.nih.govDrugBank Online – Pirtobrutinib
https://go.drugbank.comNational Center for Biotechnology Information (NCBI) – Small Molecule Resources
https://www.ncbi.nlm.nih.govRCSB Protein Data Bank – Kinase Structure Resources
https://www.rcsb.orgWikipedia – Pirtobrutinib (Chemical Overview)
https://en.wikipedia.org
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1 review for Pirtobrutinib 100mg | High purity | Factory manufactured
Q1: What is Pirtobrutinib used for in laboratory research?
A1: Pirtobrutinib is primarily used for in vitro molecular mechanism studies, kinase interaction assays, and signal pathway exploration under controlled laboratory conditions.
Q2: Can Pirtobrutinib be used in animal or clinical studies?
A2: No. This product is strictly for laboratory research and in vitro applications. It is not intended for animal, human, or clinical use.
Q3: What is the purity of Pirtobrutinib?
A3: Pirtobrutinib is supplied at ≥99% purity, verified by HPLC, ensuring reliable performance in molecular and biochemical assays.
Q4: How should Pirtobrutinib be stored?
A4: Store in a tightly sealed container under controlled laboratory conditions, protected from moisture, light, and incompatible chemicals to maintain stability.
Q5: Is Pirtobrutinib suitable for enzyme activity assays?
A5: Yes, it is widely used in cell-free enzymatic and kinase activity assays for evaluating molecular binding, affinity, and pathway modulation.
Q6: What solvents are compatible with Pirtobrutinib?
A6: Pirtobrutinib is soluble in DMSO and selected laboratory-grade organic solvents. Researchers should confirm compatibility for specific assay systems.
Q7: Can Pirtobrutinib be used for signal transduction studies?
A7: Yes, it is frequently applied in in vitro pathway reconstruction models to analyze signal propagation, feedback regulation, and interaction dynamics.
Q8: Is bulk or wholesale supply available?
A8: Yes, we provide factory-manufactured bulk supply and low-price wholesale options, suitable for long-term research projects.
Q9: Are certificates of analysis (COA) provided?
A9: Each batch comes with a COA, HPLC purity data, and analytical documentation to ensure research-grade quality and reproducibility.
Q10: Can Pirtobrutinib be used in computational or docking studies?
A10: Yes, it is compatible with in silico modeling, molecular docking, and SAR simulations to support multi-omic and computational research integration.
Q11: How should I handle Pirtobrutinib safely in the lab?
A11: Use appropriate PPE, work in a well-ventilated environment, minimize dust exposure, and follow institutional chemical safety protocols.
Q12: Is Pirtobrutinib suitable for high-throughput screening (HTS) assays?
A12: Yes, its high purity and batch consistency make it suitable as a reference compound in HTS and screening-oriented research models.
Q13: Can Pirtobrutinib be customized for purity or packaging?
A13: Yes, customization services include bulk packaging, purity adjustment, and documentation support for research-specific needs.
Q14: How do I ensure reproducibility using Pirtobrutinib?
A14: Incorporate proper controls, replicate measurements, and standardized assay conditions, along with consulting batch-specific analytical data.















manuelarana –
I’ve been looking for it for a long time, thank you