No products in the cart.
Sale
Pramlintide (Acetate)Wholesale of high-purity lyophilized powder | GMP Manufacturer
Original price was: $42.00.$36.00Current price is: $36.00.
Pramlintide is a synthetic analog of human amylin supplied as high-purity lyophilized powder for research use. It is widely studied in metabolic regulation, glucose homeostasis, and endocrine signaling research models.
Description
Product Description
Pramlintide is a synthetic peptide analog of human amylin (islet amyloid polypeptide, IAPP), engineered to improve solubility and stability compared to the native hormone. It is typically supplied as the acetate salt in lyophilized powder form and is intended strictly for research and laboratory use.
In experimental research, Pramlintide is extensively used to investigate amylin receptor signaling, gastric emptying regulation, appetite control pathways, and glucose metabolism. Due to its well-defined sequence and reproducible physicochemical properties, Pramlintide serves as a standard research peptide in metabolic, endocrine, and diabetes-related studies.

Product Specifications
| Item | Description |
|---|---|
| Product Name | Pramlintide |
| Synonyms | Pramlintide Acetate, Amylin Analog |
| CAS Number | 196078-30-5 |
| Molecular Formula | C171H267N51O53 (acetate salt) |
| Molecular Weight | ~3949.4 g/mol (acetate form, approx.) |
| Amino Acid Sequence | KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY |
| Peptide Type | Synthetic amylin analog peptide |
| Form | Lyophilized powder |
| Purity | ≥98% (HPLC) |
| Strength Options | 5 mg / 10 mg / 20 mg |
| Appearance | White to off-white powder |
| Identity Verification | HPLC, Mass Spectrometry |
| Solubility | Soluble in water (acidified aqueous solutions recommended) |
| Packaging | Sterile, sealed research vials |
| Storage Condition | −20 °C, dry and light-protected |
| Intended Use | Research use only (RUO) |
Applications
Pramlintide is commonly used in the following research areas:
Amylin receptor signaling and pathway studies
Glucose homeostasis and metabolic regulation research
Gastric emptying and satiety mechanism studies
Endocrine and pancreatic hormone interaction models
In vitro and in vivo metabolic disease research models

pramlintide Chemical structure
Usage & Reconstitution
Pramlintide lyophilized powder should be handled using standard sterile laboratory techniques. Reconstitution is typically performed in sterile water or mildly acidic aqueous buffers to enhance solubility.
Prepared solutions should be aliquoted where appropriate to avoid repeated freeze–thaw cycles. Experimental concentrations should be optimized according to specific research objectives and model systems.
Storage & Stability
Store unopened vials at −20 °C
Protect from moisture and direct light
Avoid repeated freeze–thaw cycles after reconstitution
Properly stored aliquots maintain stability for extended research use
Shipping Guarantee
Pramlintide lyophilized powder is packaged according to standardized laboratory material handling procedures:
Moisture- and light-protected inner packaging
Secure outer packaging to prevent physical damage
Batch-level labeling for traceability
Cold-chain handling applied when necessary
Contact & Bulk Inquiry
For inquiries regarding Pramlintide high-purity lyophilized powder, including bulk supply, specification details, and research support, please contact our team through the inquiry form or designated communication channels.
Our team can assist with:
Bulk and wholesale supply options
Available specifications and batch consistency
Technical documentation requests (COA, HPLC, MS)
International shipping and logistics coordination
Disclaimer
This product is intended for research and laboratory use only.
Not for human or veterinary use.
All handling and experimental applications must comply with applicable laws and institutional regulations.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |
1. What is Pramlintide mainly used for in research?
It is mainly used to study amylin signaling and metabolic regulation mechanisms.
2. Is Pramlintide suitable for glucose metabolism studies?
Yes, it is widely used in glucose homeostasis and metabolic research models.
3. What purity level is provided?
The peptide is supplied with a purity of ≥98%, verified by HPLC.
4. How is the identity of Pramlintide confirmed?
Identity is confirmed by HPLC and mass spectrometry analysis.
5. How should Pramlintide be reconstituted?
It is typically reconstituted in sterile water or mildly acidic buffers to improve solubility.
6. What storage conditions are recommended?
Store at −20 °C, protected from moisture and light.
7. Can Pramlintide be used in animal research models?
Yes, it is commonly studied in various experimental metabolic and endocrine models.
8. Is this product intended for clinical use?
No. It is strictly intended for research and laboratory use only.
9. What factors should be considered when designing experiments?
Peptide concentration, model selection, and solution stability should be carefully evaluated.
10. Is bulk supply available for research institutions?
Yes, multiple package sizes are available to support bulk research requirements.




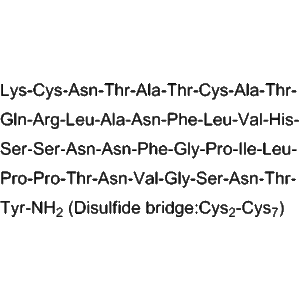














Reviews
There are no reviews yet.