No products in the cart.
Sale
(R)-Zadavotide guraxetan | CAS No. 2647371-60-4 manufacturer
Original price was: $36.00.$23.00Current price is: $23.00.
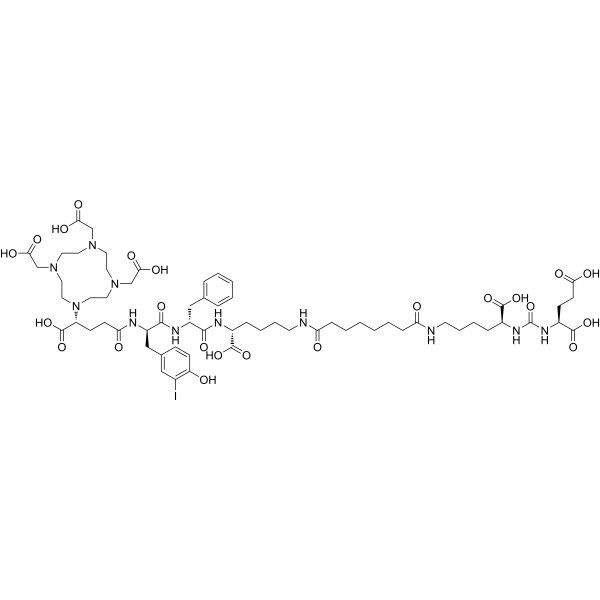
(R)-Zadavotide guraxetan (PSMA I&T; PNT-2002) is a prostate-specific membrane antigen (PSMA) inhibitor. It exhibits strong tumor-targeting properties and plays an important role in prostate cancer therapy research, imaging, and radionuclide-drug development.
Description
1. Product Description
(R)-Zadavotide guraxetan (PSMA I&T; PNT-2002) represents one of the most advanced tools in the field of oncology research, molecular imaging, and theranostics. It has been specifically designed to target the prostate-specific membrane antigen (PSMA), a transmembrane glycoprotein that plays a critical role in prostate cancer biology.
Background on PSMA and Its Relevance
PSMA is overexpressed in prostate cancer cells, particularly in advanced, castration-resistant, and metastatic prostate cancer (mCRPC). Importantly, it shows limited expression in most normal tissues, except for the kidneys, salivary glands, and certain epithelial tissues. This differential expression provides a therapeutic window that allows PSMA inhibitors such as Zadavotide guraxetan to selectively target tumors while minimizing damage to healthy tissue.
Over the past decade, PSMA-targeting ligands have revolutionized both diagnostic imaging and therapeutic strategies. For instance, PSMA-based PET tracers are now widely used for the detection of micro-metastases that cannot be easily identified using conventional imaging modalities. Likewise, radiolabeled PSMA inhibitors are at the forefront of targeted radionuclide therapy, where they deliver isotopes such as 177Lu or 225Ac directly to tumor sites.
Zadavotide guraxetan in Research
Zadavotide guraxetan is a second-generation PSMA inhibitor that combines high binding affinity, improved pharmacokinetics, and suitability for radiolabeling. It has become a backbone in research programs exploring:
PSMA-targeted PET/CT imaging using isotopes like 68Ga or 18F.
Radionuclide therapy with therapeutic isotopes such as 177Lu or 225Ac.
Theranostic platforms that integrate both diagnostic and therapeutic functions in a single molecule.
Combination cancer therapies where PSMA-targeted agents are paired with chemotherapy, immunotherapy, or hormonal therapy.
Advantages of Zadavotide guraxetan
High specificity – strong binding to PSMA overexpressed in tumors.
Versatility – can be radiolabeled with both diagnostic and therapeutic isotopes.
Improved stability – stable in physiological environments, ensuring reliable research outcomes.
Proven research utility – widely used in academic, translational, and pharmaceutical studies.
Research Applications
Oncology: Understanding mechanisms of prostate cancer progression.
Molecular Imaging: Enabling high-resolution PET scans.
Theranostics: Bridging diagnosis and therapy.
Drug Development: Serving as a template for new PSMA inhibitors.
2. Product Specifications
Below is a detailed table outlining the physicochemical and biological attributes of (R)-Zadavotide guraxetan.
| Parameter | Details |
|---|---|
| Product Name | (R)-Zadavotide guraxetan |
| Synonyms | PSMA I&T; PNT-2002 |
| CAS No. | 2647371-60-4 |
| Molecular Type | Synthetic peptide inhibitor |
| Target | Prostate-specific membrane antigen (PSMA) |
| Mechanism | High-affinity PSMA binding; tumor-specific targeting |
| Applications | Prostate cancer research, PET imaging, radionuclide therapy |
| Molecular Weight | Approx. 1500–2000 Da (depending on salt form) |
| Purity | ≥98% (HPLC validated) |
| Appearance | White to off-white lyophilized solid |
| Solubility | Soluble in PBS, water, DMSO |
| Stability | Stable ≥12 months at -20°C |
| Storage | -20°C, desiccated, away from light |
| Delivery Form | Lyophilized powder, sealed vials |
| Radiolabeling Compatibility | 68Ga, 18F, 177Lu, 225Ac, etc. |
| Quality Control | HPLC, Mass spectrometry, NMR validated |
Extended Notes on Specifications
Purity and Stability: With purity levels ≥98%, the compound ensures minimal experimental variability. Lyophilized formulations enhance shelf life and allow long-term storage.
Solubility: Versatile solubility profile makes it suitable for both in vitro assays and in vivo preclinical studies.
Radiolabeling Potential: A key advantage is its compatibility with multiple isotopes. Researchers can adapt it for diagnostic PET tracers or therapeutic agents.
Batch Consistency: QC testing guarantees reproducibility across experiments, which is essential for translational research.
3. Mechanism of Action
The action of (R)-Zadavotide guraxetan revolves around its specific and high-affinity binding to PSMA, leading to selective tumor targeting.
Stepwise Mechanism
Target Recognition: Zadavotide guraxetan identifies PSMA on the surface of prostate tumor cells.
Binding Affinity: Through its optimized molecular scaffold, it establishes high-affinity interactions with the PSMA active site.
Internalization: The PSMA-ligand complex undergoes receptor-mediated endocytosis, allowing the compound to accumulate inside tumor cells.
Payload Delivery: When radiolabeled, the compound delivers radioisotopes directly into tumor cells.
Cellular Effects: Depending on isotope type:
Diagnostic isotopes (e.g., 68Ga, 18F) provide PET imaging signals.
Therapeutic isotopes (e.g., 177Lu, 225Ac) emit cytotoxic radiation that induces apoptosis.
Broader Mechanistic Implications
Theranostics: Zadavotide guraxetan bridges imaging and therapy, creating a cycle where diagnosis informs treatment decisions.
Combination Potential: By pairing with chemotherapy or immunotherapy, researchers can explore synergistic effects.
Selectivity Advantage: High tumor-to-background uptake improves both imaging clarity and therapeutic safety margins.
Clinical Relevance in Research
Clinical trials using PSMA-targeted ligands have demonstrated:
Significant improvement in detection of recurrent prostate cancer.
Enhanced progression-free survival in mCRPC patients receiving PSMA-targeted radionuclide therapy.
Potential extension of these ligands into non-prostate tumors that also express PSMA, such as renal cell carcinoma and glioblastoma.

4. Side Effects
Although Zadavotide guraxetan itself is a research-use-only compound, its radiolabeled analogs have been studied in preclinical and clinical contexts. The side effects noted in these settings provide insights into its biological behavior.
Documented and Potential Side Effects
Renal Uptake – Kidneys exhibit PSMA expression, leading to off-target accumulation. Potential nephrotoxicity has been observed.
Salivary Gland Uptake – Radiolabeled forms accumulate in salivary glands, sometimes causing xerostomia (dry mouth).
Bone Marrow Suppression – Depending on isotope type, extended therapy may reduce hematopoietic activity.
Fatigue and GI Disturbances – Nausea, vomiting, and loss of appetite have been reported.
Radiation Toxicity – With therapeutic isotopes, localized radiation exposure can damage non-target tissues.
Laboratory Safety Notes
Use appropriate radiation shielding when working with radiolabeled derivatives.
Store compounds under recommended safety protocols.
Dispose of waste according to institutional biosafety and radiation safety guidelines.
Long-Term Considerations
Research is ongoing into PSMA-targeting nephroprotection strategies, such as co-infusion with amino acids to reduce renal uptake.
Xerostomia management remains a topic of active clinical study, with potential supportive interventions including sialoprotective agents.
Disclaimer
For laboratory research use only. Not for human, veterinary, or clinical use.
Keywords
(R)-Zadavotide guraxetan, CAS No. 2647371-60-4, PSMA I&T, PNT-2002, prostate cancer inhibitor, PSMA-targeted peptide, PET imaging agent, radionuclide therapy research.
Shipping Guarantee
Worldwide shipping with professional customs support.
Secure packaging ensures integrity during transit.
Compensation provided for damaged/lost shipments.
Transaction Guarantee
Flexible secure payments: T/T, PayPal, Crypto.
Transparent order tracking.
Buyer protection on all transactions.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 56 × 23 × 56 cm |
1 review for (R)-Zadavotide guraxetan | CAS No. 2647371-60-4 manufacturer
1. What is (R)-Zadavotide guraxetan?
A PSMA-targeted inhibitor for prostate cancer research.
1. What is (R)-Zadavotide guraxetan?
A PSMA-targeted inhibitor for prostate cancer research.
2. What are its synonyms?
PSMA I&T, PNT-2002.
3. What is its CAS number?
2647371-60-4.
4. What are its applications?
Used in prostate cancer research, PET imaging, and theranostic studies.
5. Is it radiolabeled?
Not inherently, but commonly studied in radiolabeled forms.
6. How should it be stored?
At -20°C, away from light and moisture.
7. What is its purity?
≥98%, HPLC validated.
8. Does it have clinical approval?
No, it is for research use only.
9. What makes it important for prostate cancer studies?
Its ability to selectively target PSMA, a biomarker overexpressed in prostate tumors.
10. Can it be used for other cancers?
Emerging studies suggest potential in PSMA-expressing non-prostate tumors.
11. What are possible side effects?
Renal uptake, xerostomia, bone marrow suppression (mainly in radiolabeled forms).
12. How does it differ from other PSMA ligands?
Improved pharmacokinetics, versatility in radiolabeling, and proven theranostic applications.















Pablo –
The product quality fully met our expectations for research-grade materials, with high purity and consistent batch performance.