No products in the cart.
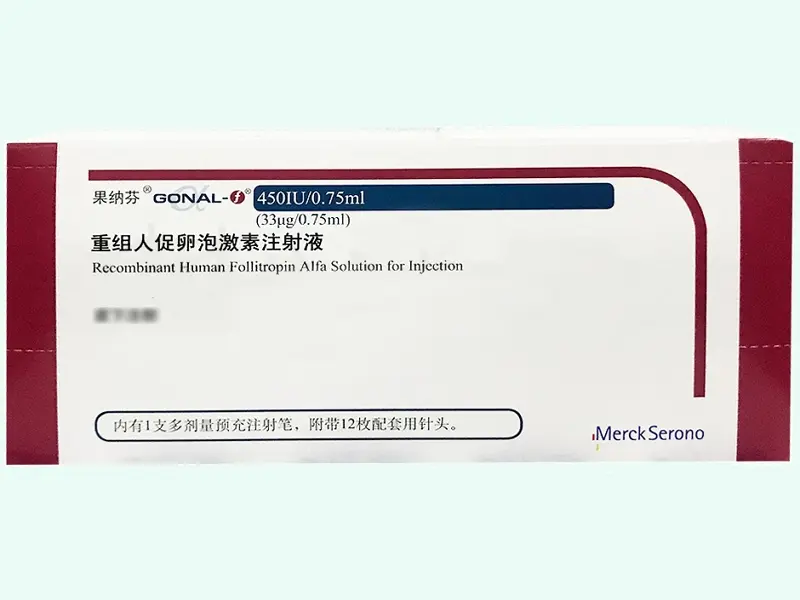
Recombinant FSH Injection Gonal-F 450 IU (Follitropin Alfa) – Pre-filled Pen
$1.00
Gonal-F (Follitropin Alfa) is a recombinant human follicle-stimulating hormone (r-hFSH) designed for laboratory-based reproductive and endocrine studies. This product comes in a 33?g (450 IU) pre-filled injection pen and is manufactured by Merck Serono S.p.A., Italy.
Approved under NMPA SJ20160040, Gonal-F is widely used in ovarian stimulation research, hormone response modeling, and in vitro fertilization (IVF) protocol simulation.
?? Strictly for research use only. Not intended for human or veterinary treatment.
Description
Recombinant FSH Injection Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Recombinant FSH Injection (Gonal-F) |
| Generic Name | Follitropin Alfa |
| Strength | 33 ?g (450 IU) per pre-filled pen |
| Dosage Form | Injection (pre-filled pen) |
| Packaging | 1 Pen / Box |
| Approval No. | SJ20160040 (NMPA, China) |
| Pharmaceutical Code | 86978860000259 |
| Manufacturer | Merck Serono S.p.A, Italy |
| CAS Number | 9002-68-0 |
| Storage Conditions | Store at 2–8°C. Protect from light. Do not freeze. |
| Intended Use | For laboratory research only |
Recombinant FSH Injection Mechanism of Action
Follitropin Alfa (rFSH) mimics the biological activity of natural FSH by binding to FSH receptors in the ovary, stimulating follicular development. In research settings, it is used to study ovulation induction, ovarian physiology, and hormone-dependent cellular responses.
Research Applications
In vitro ovarian stimulation protocols
Fertility hormone cycle modeling
Endocrine axis pathway analysis
IVF and ART preclinical models
Humanized animal models for endocrine function
Pharmacokinetic studies of recombinant hormones
Safety and Handling
Observed Lab Effects:
Local injection-site reactions
Hormonal fluctuation (temporary)
Mild inflammatory responses (in vivo)
Handling Instructions:
Use sterile lab equipment
Avoid exposure to heat and light
Wear appropriate PPE (gloves, mask, goggles)
Dispose of used pens in accordance with lab biohazard waste protocol
?? Research-Use Disclaimer
This product is intended strictly for laboratory research purposes only. It is not approved for human consumption, medical diagnosis, therapeutic use, or veterinary application. Unauthorized usage may pose safety risks and violate local regulations.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 16 × 18 × 16 cm |
Q1: Is Gonal-F 450 IU Injection approved for human use?
A: No. This version is strictly for laboratory research and not intended for clinical or therapeutic applications.
Q2: What’s the CAS number of Follitropin Alfa in this Gonal-F product?
A: The CAS number is 9002-68-0.
Q3: Can Gonal-F 450 IU be used for in vivo animal testing?
A: Yes, if approved by relevant ethics committees. This product supports both in vitro and in vivo endocrine studies.
Q4: Who manufactures this injection?
A: It is manufactured by Merck Serono S.p.A., Italy, a leader in biotechnology.
Q5: How should I store the pre-filled pen?
A: Store at 2–8°C, do not freeze. Keep away from direct sunlight.












Reviews
There are no reviews yet.