Recombinant Human G?CSF Injection 150?µg
$2.00
Recombinant human granulocyte colony–stimulating factor (G?CSF, brand: Lishengsu®) is a filgrastim formulation produced by Beijing SL Pharmaceutical Co., Ltd. (NMPA No.?S19980010; Code?86900148000828). Supplied as 150?µg solution in vials, 5 vials per box, barcode 6944580400383. Used to promote neutrophil recovery in experimental models. Strictly for laboratory research?Please consult the staff for other purposes?
描述
Recombinant Human G?CSF Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Recombinant Human G?CSF Injection (Lishengsu®) |
| Active Ingredient | Filgrastim (recombinant human G?CSF) |
| Strength | 150?µg per vial |
| Quantity | 5 vials per box |
| Dosage Form | Injectable solution (non-sterile, for research use) |
| Approval No. | China NMPA S19980010 |
| Product Code | 86900148000828 |
| Barcode | 6944580400383 |
| Manufacturer | Beijing SL Pharmaceutical Co., Ltd. |
| Intended Use | Laboratory research only — not for clinical or veterinary use |
Mechanism of Action
Filgrastim is a recombinant form of human G?CSF that binds the G?CSF receptor on hematopoietic progenitors, activating JAK/STAT and MAPK pathways. It stimulates proliferation, differentiation, and release of neutrophils from bone marrow.
Research & Pharmacology Context
-
G?CSF is widely used in experimental models to reverse chemotherapy-induced neutropenia and to aid bone marrow transplant recovery.
-
Short-acting form (filgrastim) clears in about 3.5?h post subcutaneous injection; primarily excreted via kidneys in animal studies.
-
G?CSF also displays neuroprotective effects in preclinical stroke and spinal cord injury models (activates AKT/ERK signaling).
pmc.ncbi.nlm.nih.gov+2pmc.ncbi.nlm.nih.gov+2link.springer.com+2 -
Meta-analyses in Chinese patients show long-acting G?CSF offers similar efficacy and reduced severe neutropenia compared to short-acting forms.
journals.lww.com
Research Applications
-
Preclinical chemotherapy-induced neutropenia models
-
Bone marrow transplant engraftment studies
-
JAK/STAT and MAPK pathway activation research
-
Neuroprotection in stroke/spinal cord injury models
-
Comparative pharmacokinetics of short- vs long-acting G?CSF preparations
?? Research?Use Only Disclaimer
This product is provided strictly for laboratory research and development use only. Not for clinical, diagnostic, therapeutic, or veterinary applications.
其他信息
| 重量 | 1.1 公斤 |
|---|---|
| 尺寸 | 18 × 16 × 18 厘米 |





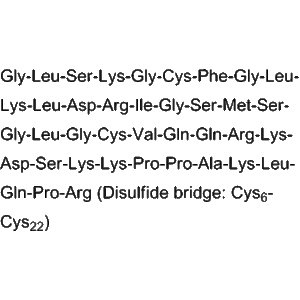
评价
目前还没有评价