No products in the cart.
Sale
Reltecimod TFA (AB-103 TFA) – High Purity 99.78% CD28 Antagonist Peptide – GMP Manufacturer
Original price was: $15.00.$13.00Current price is: $13.00.
Reltecimod TFA (AB-103 TFA) is a synthetic CD28-targeting peptide (purity 99.78%) that modulates inflammation by attenuating the CD28/B7-2 pathway. It offers protective effects against infections, exotoxins, endotoxins, and radiation. Designed for necrotizing soft-tissue infection and immunomodulation research, available for wholesale and retail.?For wholesale prices, other specifications and uses, please consult our staff?
Description
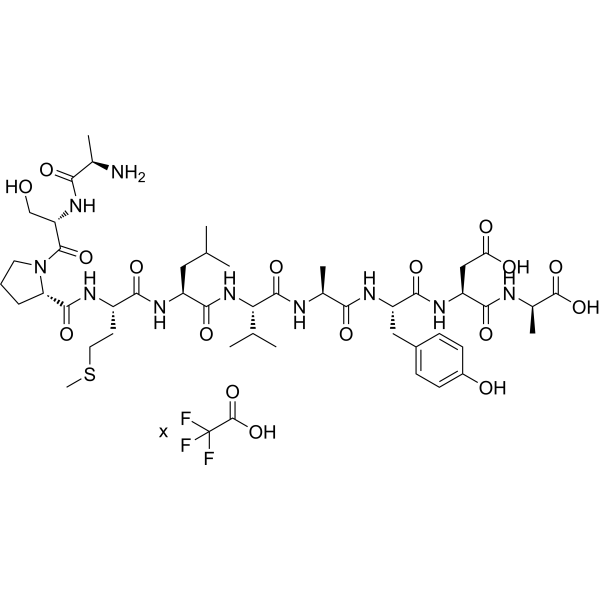
Reltecimod TFA, also known by its investigational code AB-103, is a synthetic peptide antagonist of the T-cell co-stimulatory receptor CD28 (TP44). Crafted under GMP-compliant processes, this peptide—boasting 99.78% purity—has demonstrated potent modulation of immune responses in preclinical and clinical studies. It attenuates the CD28/B7-2 co-stimulatory signaling axis, helping to mitigate excessive inflammatory cascades while preserving necessary immune function.
In preclinical murine infection models, a single dose of Reltecimod significantly increased survival, lowered pro-inflammatory cytokine surges, and improved outcomes compared to repetitive dosing.
In a Phase 3 clinical trial involving patients with necrotizing soft-tissue infections (NSTI), early administration of Reltecimod enhanced resolution of organ dysfunction and improved clinical outcomes, particularly in per-protocol analysis.
Reltecimod TFA Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Reltecimod TFA (AB-103 TFA) |
| Synonyms / Class | AB-103 TFA; CD28 antagonist peptide |
| Purity | 99.78% (HPLC, GMP-grade) |
| Form | Lyophilized powder |
| Appearance | White to off-white crystalline solid |
| Molecular Formula | C??H??N??O??S × C?HF?O? |
| Molecular Weight | 1037.19 (free base) |
| Solubility | High in DMSO; moderate in aqueous buffers |
| Stability / Storage | Store at –20 °C, protected from moisture and light |
| Manufacturing Standard | GMP-compliant synthesis and purification |
| Applications | NSTI research, infection models, immunomodulation |
| Regulatory Status | For laboratory research use only—non-clinical |
Reltecimod TFA Mechanism of Action & Research Applications
Mechanism of Action
Reltecimod targets CD28/B7-2 co-stimulation on T cells, moderating T-cell activation without full inhibition. This fine-tuned modulation helps dampen overwhelming cytokine responses to bacterial pathogens, exotoxins, endotoxins, and radiation-induced injury.
Research Applications
Necrotizing Soft-Tissue Infection (NSTI) Models — Promotes resolution of organ dysfunction and improved clinical outcomes when administered early.
Preclinical Infection Studies — Single-dose regimens in lethal infection models yield improved survival and reduced cytokine storm.
Inflammation Modulation — Provides a tool to study immune attenuation mechanisms in models of septic shock, bacterial infection, and radiation injury.
Immunological Signaling Research — Unravels the balance between T-cell activation and suppression via CD28-dependent pathways.
Reltecimod TFA Development & Formulation Notes
Reconstitution: Dissolve in DMSO to prepare concentrated stocks (e.g., 5–10 mg/mL). Dilute into buffer immediately before use.
Storage & Aliquoting: Store aliquots at –80 °C for long-term stability; avoid multiple freeze-thaw cycles.
Assay Guidance: In infection and organ dysfunction models, proactive administration (e.g., within 6 hours post-diagnosis) yields optimal outcomes. Use single-dose protocols for best efficacy, per preclinical findings.

Disclaimer
Reltecimod TFA is supplied strictly for laboratory research use only. It is not a pharmaceutical, therapeutic agent, or diagnostic product and is not approved for human or veterinary use. Handle under proper biosafety protocols and dispose of materials according to local regulations. Users are responsible for confirming suitability and compliance within their research settings.
Reltecimod TFA Packaging & Availability
Standard Packs: 2 mg, 5 mg, or 10 mg
Bulk/Wholesale: Multi-gram availability with batch consistency and reserved supply
Each shipment includes a Certificate of Analysis (CoA) verifying purity, identity, and handling guidelines.
Quality Assurance
Manufactured under strict GMP standards. Each batch is validated using mass spectrometry (MS), high-performance liquid chromatography (HPLC), and stability tests. Lot-specific documentation is provided to support research integrity and possible audit requirements.
Custom Services
Aliquoting & Custom Labeling: Fitment for LIMS or ELN systems.
Formulation Support: Guidance on buffer systems and DMSO-based stock solutions.
Stability Study Design: Consultation for storage and transportation planning.
Experimental Strategy Support: Optimization for NSTI, cytokine storm, or immunomodulatory models.
Keywords
Reltecimod TFA; AB-103 TFA; CD28 antagonist peptide; necrotizing soft tissue infection research; NSTI immunomodulator; cytokine storm peptide; GMP peptide; CD28/B7-2 pathway; infection model peptide; research-use-only.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 85 × 82 × 85 cm |
What is Reltecimod TFA?
A synthetic CD28-targeting peptide (AB-103 TFA) that modulates immune activation without complete inhibition.
What purity does it have?
99.78% purity via HPLC, GMP-manufactured.
What is its primary research use?
Studying inflammatory responses and organ dysfunction in necrotizing soft-tissue infections.
Is it used clinically?
No—it is strictly a research-use-only reagent.
How should it be stored?
Store lyophilized at –20 °C; reconstituted stocks at –80 °C, avoiding freeze-thaw cycles.
What animal models are relevant?
Mouse models of lethal infection and NSTI; clinical trial data supports translational relevance
What is the optimal dosing regimen?
Preclinical data supports a single intravenous dose within 1–2 hours of infection for best survival outcomes
How does it compare to repeated dosing?
A single dose was superior in survival and cytokine suppression compared to multiple dosing
Is documentation provided?
Yes—each lot includes a CoA with purity, identity, and quality data.
Can you support custom experimental setups?
Yes—we offer formulation, stability testing, and research design assistance per scientific need.













Reviews
There are no reviews yet.