No products in the cart.
Sale
Rusfertide Acetate – Purity 99.83% | GMP Factory
Original price was: $68.00.$53.00Current price is: $53.00.
Rusfertide Acetate is a synthetic peptide supplied as a lyophilized powder for laboratory research use. It is commonly studied in peptide signaling, metabolic regulation, and molecular interaction research models.
Description
Applications
Rusfertide Acetate is intended for use exclusively in laboratory and scientific research environments. Common research applications include:
Peptide signaling pathway and molecular interaction studies
Evaluation of peptide structure–activity relationships (SAR)
Metabolic regulation research using experimental peptide models
Stability and formulation studies of synthetic peptide compounds
Analytical method development for peptide identification and purity assessment

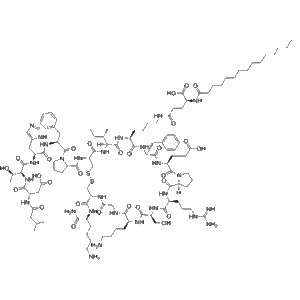
Rusfertide Acetate Chemical structure
Product Description
Rusfertide Acetate is a synthetic peptide developed for research applications involving peptide-mediated signaling and metabolic regulatory mechanisms. In laboratory settings, it is primarily used to explore peptide structure, molecular interactions, and experimental behavior under controlled conditions.
Supplied as a lyophilized powder, Rusfertide Acetate offers enhanced stability for storage and handling in research environments. All studies involving this compound are strictly limited to non-clinical and non-therapeutic laboratory research.
Product Specifications
| Item | Description |
|---|---|
| Product Name | Rusfertide Acetate |
| Synonyms | PTG-300 |
| CAS Number | Not officially assigned |
| Molecular Formula | C₁₆₈H₂₆₄N₄₆O₅₄ · xC₂H₄O₂ |
| Molecular Weight | ~2100 g/mol (peptide, approximate) |
| Amino Acid Sequence | Proprietary / research reference |
| Peptide Type | Synthetic peptide |
| Form | Lyophilized powder |
| Purity | ≥98% (HPLC) |
| Strength Options | 5 mg, 10 mg, 20 mg |
| Appearance | White to off-white lyophilized powder |
| Identity Verification | HPLC, MS |
| Solubility | Soluble in water or suitable laboratory buffers |
| Packaging | Sterile vial |
| Storage Condition | −20 °C, protected from light and moisture |
| Intended Use | Laboratory research use only |
Storage & Stability
Rusfertide Acetate should be stored at −20 °C in a dry and light-protected environment. When maintained as a lyophilized powder under recommended conditions, it demonstrates good stability for research use. Reconstituted solutions should be handled according to validated laboratory protocols.
Contact & Research Inquiry
For technical specifications, batch documentation, or research-related inquiries regarding Rusfertide Acetate lyophilized raw materials, please contact our team. Information is provided to qualified research and laboratory institutions upon request.
Disclaimer
This product is intended for laboratory research use only. It is not intended for human or veterinary use and must not be used for diagnostic, therapeutic, or clinical purposes. All information provided is for scientific reference only.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 28 × 22 × 28 cm |
1. What is Rusfertide Acetate mainly used for in research?
It is used in laboratory studies focused on peptide signaling mechanisms and molecular interaction analysis.
2. Is Rusfertide Acetate also known as PTG-300?
Yes. PTG-300 is a commonly used research identifier for Rusfertide.
3. Why is Rusfertide Acetate supplied as a lyophilized powder?
Lyophilization improves stability and supports long-term storage for laboratory research.
4. What analytical techniques are used to verify product quality?
HPLC and mass spectrometry are typically used for identity and purity verification.
5. Can Rusfertide Acetate be used in cell-based research models?
It may be used in approved in vitro experimental systems following proper laboratory protocols.
6. How is Rusfertide Acetate usually reconstituted for experiments?
It is commonly dissolved in sterile water or compatible laboratory buffers.
7. Is Rusfertide Acetate suitable for long-term research storage?
Yes, when stored at −20 °C as a lyophilized powder, it remains stable.
8. Is Rusfertide Acetate approved for clinical or therapeutic use?
No. It is not approved for clinical, therapeutic, or diagnostic use.
9. Is this product intended for human or veterinary use?
No. This product is strictly intended for laboratory research use only.
10. Are different research-scale quantities available?
Yes. Multiple laboratory-scale quantities are available to support diverse research needs.















Reviews
There are no reviews yet.