No products in the cart.
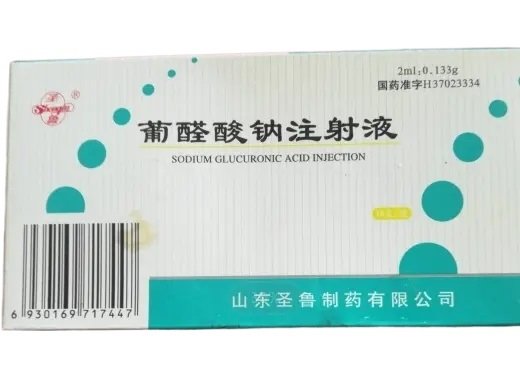
Sodium Glucuronate Injection 0.133?g – Low Price Wholesale Comparison
$1.00
This Sodium Glucuronate Injection delivers 133?mg of sodium glucuronic acid per 2?ml sterile vial, with 10 vials per box. Manufactured by Shandong Shenglu Pharmaceutical Co., Ltd., and approved under NMPA H37023334. It is a research-grade hepatoprotective agent used in models of hepatitis, cirrhosis, detoxification, and oxidative liver injury.
?? For laboratory and research use only.?Please consult staff for other specifications and uses?
Description
Sodium Glucuronic Acid, the sodium salt of D-glucuronic acid, enhances hepatic detox pathways by binding toxins to form glucuronides, promoting safe excretion. It helps increase liver glycogen, reduce lipolysis, and protect against liver injury induced by toxins or diseases such as chronic hepatitis and cirrhosis. The sterile injection form allows for precise dosing in preclinical hepatoprotection studies.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Sodium Glucuronate Injection |
| Generic Name | Sodium Glucuronic Acid (D?Glucuronic Acid Sodium Salt) |
| CAS Number | 14984?34?0 |
| Molecular Formula | C?H??O?Na |
| Molecular Weight | ~234.25?g/mol |
| Formulation | Clear sterile aqueous solution |
| Strength per Vial | 0.133?g glucuronic acid per 2?ml |
| Pack Size | 10 vials per box |
| Dosage Form | Injection (solution) |
| Approval Number | H37023334 |
| Drug Standard Code | 86904207000569 |
| Manufacturer | Shandong Shenglu Pharmaceutical Co., Ltd. |
| Storage Conditions | Store protected from light and moisture; below 25?°C |
| Intended Use | Lab research: hepatoprotection, detoxification, toxic liver injury |
Mechanism of Action
Sodium glucuronate is converted into glucuronic acid, which conjugates toxins via glucuronidation, enhancing renal/biliary excretion. It also helps preserve liver glycogen and suppress lipolysis, thus aiding detox and liver cell protection.
Research Application Areas
Preclinical models of acute and chronic hepatitis
Cirrhosis and hepatic fibrosis protection studies
Detoxification and toxin-binding assays
Oxidative stress mitigation in liver injury models
Enhancement of glucuronidation pathway biomarker research
Safety & Handling
Dosage in Animal Models: IM or IV at 0.133–0.266?g per injection, administered 1–2× daily
Adverse Effects: None reported in clinical use
Contraindications: Hypersensitivity to the compound
Storage: Keep sealed, avoid high temperatures and light exposure
Handling: Use PPE; ensure sterility
Core Keywords
sodium glucuronate injection, sodium glucuronic acid injection, CAS 14984-34-0, glucuronide hepatoprotection, H37023334, detox liver injection, research-grade glucuronate, wholesale sodium glucuronate, lab hepatoprotective agent
Research Use Disclaimer
This product is intended exclusively for laboratory and preclinical research. It is not approved for clinical, therapeutic, diagnostic, or veterinary use. Misuse may have legal or health implications. Use only under appropriate institutional protocols.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 32 × 36 × 32 cm |










Reviews
There are no reviews yet.