No products in the cart.
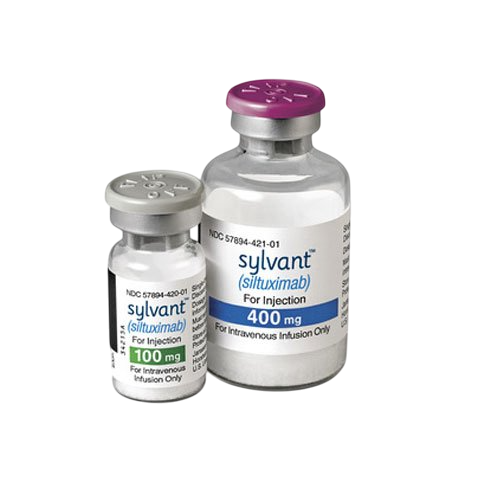
Sylvant (siltuximab) 400?mg Vial Injection – For Research Use Only
$2.00
Sylvant (siltuximab) is a chimeric monoclonal antibody that binds and neutralizes interleukin?6 (IL?6). Supplied as 400?mg injectable vial by Patheon Italia under license SJ20210032. Ideal for cytokine signaling, inflammation, and lymphoproliferative disease research. For laboratory research use only. Wholesale & retail supported.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Siltuximab (CNTO 328) is a chimeric (human–mouse) IgG1? monoclonal antibody specifically targeting IL?6. It prevents IL?6 from activating its soluble and membrane-bound receptors, reducing B?cell proliferation, VEGF secretion, and inflammatory cascades. Although clinically approved for idiopathic multicentric Castleman’s disease, it is used in research as a tool to model IL?6–driven hyperinflammation, cytokine storm, and lymphoproliferative processes. Provided as a 400?mg vial for intravenous use. For laboratory research use only.
Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Sylvant (siltuximab) Injection |
| Synonyms | Siltuximab; CNTO?328; IL?6 neutralizing antibody |
| Strength | 400?mg per vial |
| Dosage Form | Injectable vial (lyophilized or solution) |
| Packaging | 1 vial per box |
| Manufacturer | Patheon Italia S.p.A. (Switzerland-based) |
| Approval Number | SJ20210032 |
| Drug Standard Code | 86983716000031 |
| Barcode | Not yet assigned |
| CAS Number | 541502?14?1 |
| Molecular Formula | C????H????N????O????S?? |
| Molecular Weight | ~148?kDa |
Mechanism of Action & Research Applications
Siltuximab neutralizes IL?6, a central cytokine in inflammatory and lymphoproliferative pathways. Its research utility spans modeling cytokine storms, IL?6–driven disease mechanisms, VEGF-associated vascular growth, and signal transduction in hyperinflammation. Useful for cytokine pharmacodynamics, immune modulation assays, and lymphoid proliferation studies. For laboratory research use only.
Side Effects (For Reference Only in Research Models)
Clinical analog data note possible adverse events such as weight gain, rash, pruritus, hyperuricemia, upper respiratory infection, hypertension, fatigue, neutropenia, and infusion reactions. These observations inform safety and toxicity design in research dosing models.
Disclaimer
Sylvant (siltuximab) is strictly intended for laboratory research use only. Not for human or veterinary therapeutic, diagnostic, or prophylactic use.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |










Reviews
There are no reviews yet.