No products in the cart.
Toremifene Citrate 60mg for scientific research
$1.00
High-purity Toremifene Citrate Tablets designed exclusively for in vitro molecular mechanism research, receptor-binding analysis, and biochemical pathway modulation studies. Factory-manufactured with consistent batch quality, suitable for bulk and wholesale supply.(For research use only. Not for human or veterinary use.)
Description
Product Description
Toremifene Citrate Tablets are high-purity, factory-manufactured solid research materials designed specifically for in vitro molecular mechanism studies, biochemical pathway analysis, and controlled receptor-associated assays. Produced under stringent quality and material-control procedures, these tablets provide a structurally stable and compositionally consistent format suitable for laboratories performing analytical chemistry evaluations, receptor-binding characterization, and multi-layered signaling pathway exploration. Unlike variable powder forms, tablets offer enhanced uniformity, reproducibility, and ease of handling, allowing researchers to maintain controlled mass measurements and standardized dissolution workflows across repeated experiments.
Each tablet contains precisely formulated Toremifene Citrate to support research focusing on ligand–receptor interactions, transcriptional complex modulation, protein conformational analysis, and downstream molecular response mapping. This makes Toremifene Citrate Tablets an optimal choice for laboratories investigating receptor-cofactor dynamics, competitive ligand behavior, signal-transduction modulation, and pathway regulatory mechanisms. Their stable composition ensures minimal degradation under recommended storage conditions, preserving molecular integrity throughout extended research cycles.
In vitro studies frequently employ toremifene citrate tablets to examine molecular pathways affected by selective receptor modulators, enabling the characterization of protein recruitment patterns, alterations in transcription factor binding, chromatin remodeling behavior, and gene-expression signatures triggered by receptor-associated molecular events. These controlled responses provide a robust platform for integration with high-content screening, proteomic profiling, structural-bioinformatics simulations, and transcriptomic mapping workflows. The tablet format allows researchers to dissolve the material in controlled laboratory solvents and subsequently perform quantitative assays without concerns about particle-size inconsistency or density variation often encountered in raw powders.
To support industrial-scale research environments, this product is manufactured through a factory-direct process, ensuring reliable bulk and wholesale availability. Laboratories requiring consistent, large-batch quantities benefit from uniform tablet formulation, reduced variability, and comprehensive documentation suitable for internal quality tracking. Each production batch undergoes high-performance analytical verification, including purity assessment via HPLC, structural confirmation, and contaminant screening to align with high standards of research chemistry.
Toremifene Citrate Tablets integrate seamlessly into modern multi-omic experimental pipelines, including proteomics, metabolomics, chemoinformatics, and receptor-binding computational modeling. Their stable form and high purity allow researchers to build reproducible datasets suitable for systems-level pathway analysis, cross-dataset integration, and advanced mechanistic inference.
Designed strictly for laboratory use, these tablets provide a dependable, high-quality research reagent for academic laboratories, industrial R&D programs, and mechanistic discovery platforms requiring high-purity toremifene citrate tablets. Their consistent performance, secure factory-level production, and wholesale-ready supply chain make them an ideal choice for long-term research programs, analytical method development, and multi-dimensional biochemical investigation.

Product Specifications
| Parameter | Description |
|---|---|
| Product Name | Toremifene Citrate Tablets (Research Grade) |
| Form | Solid compressed tablet (laboratory research material) |
| Composition | Contains high-purity Toremifene Citrate blended with research-compatible excipients for structural stability |
| Assay Purity | ≥ 98% (HPLC verified) |
| Molecular Class | Synthetic small-molecule ligand for receptor-related pathway research |
| Tablet Integrity | Uniform compression; low friability; consistent mass distribution |
| Color & Appearance | White to off-white tablet; smooth surface; no visible particulates |
| Solubility (Research Conditions) | Soluble in appropriate laboratory solvents (e.g., analytical-grade organic solvents); solubility may vary by pH and solvent composition |
| Storage Conditions | Store in a sealed, moisture-free environment; protect from excessive heat and direct light |
| Stability | Stable under recommended lab storage; minimal degradation during controlled in vitro workflows |
| Recommended Handling | Handle with gloves; avoid exposure to humid conditions; maintain container closure when not in use |
| Intended Use | For in vitro, biochemical, and molecular mechanism research only |
| Batch Consistency | Factory-manufactured with controlled specifications for repeatable multi-batch experiments |
| Quality Verification | HPLC purity testing, structural confirmation, and contaminant screening performed on each lot |
| Packaging Options | Factory-sealed bottles or bulk laboratory containers; customizable packaging for wholesale orders |
| Scale Availability | Small research units, bulk laboratory quantities, or wholesale factory-direct supply |
| Regulatory Status | Not intended for biological administration; not for human or veterinary use |
Mechanism of Action
Toremifene Citrate Tablets function as a high-purity research material enabling controlled investigation of ligand–receptor interactions, transcriptional regulation, and complex molecular signaling pathways in strictly in vitro environments. The compound is frequently examined for its ability to interact with specific receptor families and influence protein–protein interactions, cofactor assembly, and the structural conformation of receptor-associated complexes. These properties make it highly useful in receptor-binding assays, structural biology research, and computational docking simulations where experimental reproducibility and purity are essential.
At the molecular level, toremifene citrate exhibits affinity for key receptor domains that regulate downstream signaling cascades. This interaction can lead to competitive or modulatory effects on ligand-occupied receptor states, which in turn influence chromatin remodeling, transcription-factor recruitment, and cofactor exchange dynamics. Research often focuses on the compound’s ability to alter DNA-binding motifs, disrupt or stabilize transcriptional complexes, and modulate the assembly of multi-protein regulatory structures. These mechanistic insights are particularly useful in biochemical and molecular studies seeking to map signaling networks involved in receptor-mediated transcriptional control.
In in vitro biochemical systems, Toremifene Citrate Tablets are utilized to characterize binding kinetics, receptor affinity profiles, and equilibrium interaction behaviors. Studies frequently employ surface-binding assays, fluorescence-based detection, NMR interaction profiling, and HPLC/LC–MS analytical workflows to determine how the compound influences receptor-ligand equilibrium or modulates specific conformational states. These analyses provide quantitative information about how molecular interaction patterns shift under varying concentrations, environmental parameters, or mutated receptor forms.
At the structural level, computational and crystallographic research uses toremifene citrate to explore receptor pocket geometry, hydrophobic and electrostatic interaction networks, and ligand-induced structural perturbations. Simulations often reveal how subtle shifts in ligand orientation influence receptor surface topology, protein flexibility, and allosteric communication pathways within multi-domain receptor complexes. This makes the compound highly valuable for molecular-design testing, docking-energy landscape modeling, and high-resolution structural interpretation.
Downstream of receptor engagement, research models indicate that toremifene citrate can modulate transcription-associated elements such as co-regulator loading, chromatin accessibility, and signal-dependent gene-expression patterns. These effects, studied exclusively through in vitro systems, enable scientists to map regulatory nodes, identify pathway bottlenecks, and characterize the temporal progression of molecular signaling events.
Because these Toremifene Citrate Tablets are factory-manufactured with highly consistent batch quality, researchers can reliably reproduce mechanistic observations, compare cross-batch datasets, and integrate findings across diverse multi-omic frameworks. Their stable solid-tablet form further reduces variability in mass measurement and dissolution, supporting precise quantitative mechanistic studies.
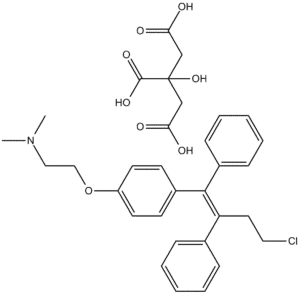
Applications
Toremifene Citrate Tablets are widely utilized in in vitro molecular mechanism research, offering a stable and precisely formulated solid form that supports advanced analytical, biochemical, and multi-omic investigations. Their controlled composition allows researchers to prepare consistent solutions for mechanistic assays without the variability typically associated with loose powder materials. These attributes make the tablets suitable for a diverse array of laboratory applications:
1. Receptor-Binding and Ligand Interaction Studies
Toremifene citrate is frequently examined as a model ligand for exploring receptor-associated molecular events. Researchers employ the tablets to study binding affinity, competitive inhibition, receptor conformational changes, and ligand-induced protein restructuring. These investigations help map key domains involved in regulatory signaling pathways and transcriptional control.
2. Transcriptional and Chromatin Modulation Research
In vitro assays exploring gene-expression regulation often use toremifene citrate to evaluate chromatin remodeling behavior, transcription-factor recruitment, and co-regulator complex dynamics. Its consistent formulation enhances reproducibility across DNA-binding, epigenomic, and chromatin-accessibility evaluations.
3. Structural Biology and Computational Modeling
Toremifene citrate is a common reference molecule in docking simulations, binding-pocket geometry analysis, allosteric modeling, and protein–ligand interaction energy profiling. The tablet format provides highly uniform starting material for experiments that integrate computational and empirical structural methods.
4. High-Content Screening and Assay Development
Laboratories design high-throughput and medium-throughput in vitro platforms using toremifene citrate to test pathway responsiveness, receptor activation states, and biochemical readouts. Its high purity supports precise control experiments, positive-reference assays, and method-validation workflows.
5. Multi-Omic Data Integration
Toremifene Citrate Tablets are increasingly used in proteomic, transcriptomic, pathway-mapping, and chemoinformatics workflows. The molecule’s well-characterized signaling interactions make it a reliable component for cross-omic comparison, dataset correlation, and algorithmic predictive modeling.
6. Material Characterization & Analytical Method Validation
Analytical chemistry laboratories utilize toremifene citrate for:
LC–MS calibration studies
Spectroscopic analysis
Stability testing
Solvent–compound interaction profiling
Its consistent purity and tablet uniformity reinforce its value as a reference material.
7. Educational and Mechanistic Demonstration Studies
University and institutional laboratories employ the tablets to demonstrate fundamental concepts in receptor biology, molecular interaction mapping, and ligand-regulated pathways under controlled in vitro conditions.
Research Models
Toremifene Citrate Tablets are widely incorporated into a variety of in vitro research models designed to investigate receptor-associated signaling, ligand–protein interactions, and transcriptional regulatory networks. Their stable tablet form provides highly consistent material suitable for reproducible scientific workflows, making them a preferred option for laboratories conducting mechanistic, structural, or analytical studies. These tablets offer researchers a reliable foundation for preparing controlled test solutions and performing multi-level biochemical analysis.
A common research model involves purified receptor-domain systems, where toremifene citrate is introduced into ligand-binding assays to observe alterations in receptor conformation, affinity behavior, and structural stability. Such systems allow precise quantification of interaction parameters, enabling scientists to map how receptor activity shifts under varying molecular conditions.
Another widely used model includes cell-free transcriptional regulation platforms, where transcription-factor complexes, DNA response elements, and co-regulator proteins are evaluated in the presence of toremifene citrate. These setups provide insight into chromatin accessibility, cofactor recruitment dynamics, and the modulation of transcriptional machinery under controlled in vitro settings.
In biochemical reconstitution assays, researchers integrate purified proteins, cofactors, and structural scaffolds to study protein–protein interaction shifts triggered by ligand-binding events. Toremifene citrate serves as a consistent modulator for analyzing cooperation or competition between signaling components.
Additionally, computational hybrid models combine empirical tablet-derived experimental data with docking simulations, molecular dynamics, and chemoinformatics workflows. Such models refine predictions on ligand orientation, binding-pocket geometry, interaction energy landscapes, and allosteric communication pathways.
High-content screening models also incorporate toremifene citrate for reference-material calibration, signal pathway benchmarking, and assay-validation processes. These include fluorescence-based assays, receptor-surface binding plates, and pathway-mapping arrays.
Because Toremifene Citrate Tablets are factory-manufactured to maintain uniformity and high purity, they fit seamlessly into long-term research programs requiring cross-batch consistency and stable analytical baselines. Their reliability enables multi-omic and structural models to operate with high reproducibility, supporting advanced discovery and mechanistic interpretation.
Experimental Design Considerations
Designing robust and reproducible studies with Toremifene Citrate Tablets requires meticulous planning across pharmacology, formulation, dosing, analytical workflows, and study controls. Below is an expanded framework to support researchers conducting in vivo, in vitro, or translational research utilizing this SERM-based oral formulation.
1. Dose Optimization & Administration Strategy
Because Toremifene citrate exhibits predictable oral bioavailability, tablets are suitable for simulating clinically relevant administration routes. However, dose selection should consider:
Species-specific metabolism (rodent vs. canine vs. primate)
First-pass hepatic conversion influencing effective plasma concentration
Tablet subdivision accuracy when fractional dosing is required
Weight-normalized dosing to maintain PK comparability across study arms
Pharmacodynamic lag time due to receptor occupancy kinetics
Where precise micro-dosing is necessary, tablets may be ground into a fine powder for suspension in aqueous vehicles, ensuring full homogenization to prevent dose stratification during gavage.
2. Study Duration & Endocrine Response Window
Toremifene’s modulation of estrogen signaling produces time-dependent effects on proliferation, apoptosis, and receptor transcriptional activity. Researchers should plan sampling timepoints to capture:
Early gene-expression shifts (1–6 hours post-dose)
Intermediate pathway effects (24–72 hours), including ERα suppression
Long-term phenotypic changes (7–28 days), such as tumor volume reduction or Ki-67 expression decline
Longitudinal designs require adherence to consistent circadian timing, as endocrine-related markers fluctuate with diurnal rhythms.
3. Control & Comparator Selection
For mechanistic clarity, studies should integrate:
Vehicle controls to distinguish SERM activity from excipient effects
Estrogen agonist/antagonist comparator groups (e.g., estradiol, tamoxifen, fulvestrant)
Sham dosing cohorts for behavioral or stress-related assessments
Positive controls where molecular readouts are known to be responsive (e.g., ER-positive tumor lines)
Including multiple comparator SERMs is particularly valuable in profiling Toremifene’s partial agonist vs. antagonist balance in tissue-specific contexts.
4. Analytical Readouts & Quantification Methods
High-quality pharmacodynamic and mechanistic interpretation requires multi-layered analytical strategies:
Receptor-binding assays to confirm ERα/ERβ occupancy
qPCR/NGS for estrogen-responsive gene panels (e.g., GREB1, PGR)
Western blot / ELISA for p-ERK, p-AKT, and apoptosis regulators
Chromatin immunoprecipitation (ChIP-seq) for mapping ER transcriptional blockade
Metabolomic profiling to assess SERM metabolism and conjugate formation
In vivo studies may further incorporate plasma drug-level monitoring via LC–MS/MS for PK–PD correlation.
5. Formulation Handling & Tablet Integrity
To preserve consistency:
Ensure uniform grinding if tablets are powdered for suspension.
Avoid high-temperature conditions that may degrade citrate salts.
Use amber containers to protect from light-sensitive degradation.
Verify dissolution uniformity when mixing with carriers like CMC-Na or methylcellulose.
Stability studies recommend preparing suspension fresh daily or storing briefly under refrigeration (<24 hours).
6. Endocrine System Confounder Control
Because Toremifene directly interacts with estrogen signaling, several confounders should be minimized:
Phytoestrogen content in animal feed
Cage materials with xenoestrogenic leachates (e.g., certain plastics)
Stress-induced cortisol changes that alter hormone feedback loops
Baseline hormonal cycles in female animal models
Synchronizing estrous cycle stages or using ovariectomized models can improve data clarity.
7. Cell Line & Animal Model Suitability
Model selection greatly influences interpretability:
ER-positive cell lines (MCF-7, T47D) for classic antagonist profiling
ER-negative lines as specificity controls
Xenografts expressing mutant ESR1 to examine endocrine resistance
Orthotopic models for metastatic behavior studies
Ovariectomized rodents to model postmenopausal endocrine environments
Toremifene’s tissue-selective effects make parallel use of two or more models recommended.
8. Drug–Drug Interaction Assessments
Toremifene undergoes hepatic metabolism involving CYP pathways; therefore:
Co-administered research compounds should be screened for CYP3A4 interactions.
Avoid excipients that may modify gastric pH excessively.
Consider multi-arm studies comparing single-agent vs. combination regimens (e.g., CDK4/6 inhibitors).
Interaction studies should include plasma-level quantification to validate additivity or antagonism.
9. Ethical & Welfare Considerations in In Vivo Studies
Maintain consistent handling to reduce stress-linked hormonal artifacts.
Use the minimum effective number of animals without compromising statistical reliability.
Monitor for endocrine-related side observations such as uterine hypertrophy or liver enzyme changes.
Tablet-based dosing generally improves welfare compared with invasive dosing regimens.
10. Statistical Design & Data Reproducibility
A solid statistical framework enhances interpretability:
Power analyses to determine appropriate cohort sizes
Randomization & blinding for treatment allocation
Longitudinal mixed-model analysis for repeated-measure timepoints
Batch-effect correction for multi-omics datasets
Standardized reporting formats following ARRIVE and NIH reproducibility guidelines
Including replication across independent batches strengthens translational relevance.
Laboratory Safety & Handling Guidelines
Safe laboratory handling of Toremifene Citrate Tablets requires controlled procedures, appropriate engineering protections, and clear risk-awareness due to the compound’s activity as a selective estrogen receptor modulator (SERM). Although supplied in a stable tablet form, research workflows involving splitting, powdering, weighing, or preparing suspensions may generate particulates or residues that pose exposure risks if not managed properly.
Researchers should wear nitrile gloves, lab coats, and protective eyewear at all times. When manipulating tablets—especially crushing or converting into powder—an N95 respirator or equivalent is recommended to minimize inhalation risk. All powder-generating procedures must be conducted inside a certified chemical fume hood or powder-containment enclosure to avoid aerosol formation and cross-contamination. Tablet splitting should be performed with a clean, dedicated device, followed by immediate surface decontamination using 70% ethanol or appropriate laboratory detergents.
Store tablets at controlled room temperature (20–25°C), away from humidity and light, and keep them sealed in their original packaging until use. Powdered preparations or laboratory-made suspensions should be clearly labeled with concentration, date, and preparer, and used promptly to avoid degradation. When preparing suspensions for in vitro workflows, ensure full homogenization and avoid prolonged standing that may cause sedimentation.
Waste materials—including contaminated PPE, wipes, residue, or expired formulations—must be disposed of as pharmaceutical or hazardous chemical waste following institutional procedures. Avoid sink disposal or placing residues in regular trash. In the event of minor spills, gently collect material using damp disposable wipes to prevent dust; major spills require activation of the laboratory’s formal chemical-spill response process.
Additional precautions apply to reproductive safety. Personnel who are pregnant, breastfeeding, or medically restricted from handling endocrine-active substances should not work directly with Toremifene. Proper documentation—including batch traceability, procedural logs, and equipment maintenance records—should be maintained to support reproducibility and compliance with internal laboratory guidelines.

Integration with Multi-Omic & Computational Studies
Toremifene Citrate Tablets are highly compatible with multi-omic research workflows and computational modeling approaches, enabling comprehensive mechanistic studies in receptor biology, transcriptional regulation, and signaling pathway analysis. Their stable tablet formulation ensures consistent concentration and purity, providing a reliable foundation for integrating experimental and computational datasets across molecular, proteomic, transcriptomic, and metabolomic platforms.
In proteomic studies, Toremifene serves as a modulator of receptor complexes, co-regulator recruitment, and downstream effector proteins. Researchers can examine changes in phosphorylation states, protein–protein interactions, or conformational dynamics in response to ligand engagement. Coupling these datasets with transcriptomic profiling allows the mapping of gene-expression patterns and identification of target genes influenced by estrogen receptor modulation under controlled in vitro conditions.
Epigenomic and chromatin-focused studies benefit from the compound’s reproducible molecular behavior, enabling high-resolution ChIP-seq, ATAC-seq, or DNA accessibility assays. Multi-layered analyses of receptor occupancy and cofactor recruitment can be cross-referenced with transcriptional readouts, providing mechanistic insights into chromatin remodeling and regulatory network modulation.
From a computational perspective, Toremifene facilitates molecular docking, ligand–receptor energy landscape modeling, and allosteric network simulations. High-purity tablets ensure that experimental input data accurately reflect true molecular interactions, improving predictive modeling accuracy. Integration with molecular dynamics simulations or in silico ligand screening enhances the interpretation of receptor conformational flexibility, binding kinetics, and thermodynamic stability.
Cross-platform data integration allows for multi-dimensional insights, combining biochemical, structural, and computational observations. Researchers can correlate ligand-induced proteomic changes with transcriptomic shifts, identify potential off-target effects, and refine pathway models. This approach supports mechanistic hypothesis generation, pathway perturbation studies, and predictive modeling in receptor-driven signaling networks.
Overall, Toremifene Citrate Tablets provide a robust and reliable reagent for multi-omic and computational research, ensuring reproducibility, batch consistency, and seamless integration into high-resolution molecular studies. Their solid, research-grade formulation facilitates precise experimental design, data comparability, and computational modeling across diverse laboratory platforms.
Things to Note
Toremifene Citrate Tablets are intended strictly for laboratory research and in vitro molecular studies.
High-purity tablets provide reliable receptor-binding and pathway-modulation data when handled according to laboratory safety guidelines.
Avoid moisture, light, and prolonged storage to maintain structural stability.
Not for human, veterinary, or clinical use.
Use only in controlled laboratory experiments, multi-omic assays, or computational modeling studies.
Keywords
toremifene citrate tablets, high-purity laboratory tablets, SERM research reagent, receptor-binding study, in vitro pathway modulator, molecular mechanism research, factory-direct bulk supply, wholesale research chemical, multi-omic research reagent, mechanistic pathway analysis, analytical chemistry tablets, Tumor (compound) Research, receptor-ligand interaction research
Shipping Guarantee
Factory-manufactured Toremifene Citrate Tablets are securely packaged to protect integrity and purity during transport. Bulk and wholesale shipments are supported with reinforced containment, moisture protection, and tracking. Each shipment is verified to ensure high-purity product delivery suitable for laboratory research.
Trade Assurance
Factory-direct low-price wholesale
Bulk customization options
Guaranteed batch consistency for research reproducibility
Payment Support
Multiple secure payment options are available for global laboratories, including PayPal, bank transfer (TT), credit cards, and cryptocurrency. Flexible payment methods support bulk, wholesale, and factory-direct procurement.
Disclaimer
Toremifene Citrate Tablets are for laboratory research use only. Not for human or veterinary use, and not intended for diagnostic, therapeutic, or clinical applications. Researchers must follow all laboratory safety protocols and handle the compound under controlled in vitro or computational study conditions.
References
National Cancer Institute. Toremifene Citrate Drug Definition. This entry describes Toremifene as a nonsteroidal SERM that binds competitively to estrogen receptors, interfering with receptor‑mediated signaling. cancer.gov
Drugs.com. Toremifene Citrate Monograph for Professionals. Summarizes the mechanism of action of Toremifene, including competitive binding at estrogen receptors, modulation of transcription pathways, and effects on gene expression. Drugs.com
Sabnis et al., Toremifene Citrate Description and In vitro Activity. Describes toremifene’s in vitro inhibition of cell growth via estrogen receptor interaction and its IC50 values in cell culture models. glpbio.com
PubMed – Review of Toremifene Pharmacological Properties. Reviews how toremifene competitively inhibits estrogen receptor binding and influences cell proliferation in culture systems. PubMed
Oxford Academic, Effects of Toremifene on Endocrine Biomarkers. Explores receptor modulation and endocrine effects in controlled research scenarios, illustrating mechanistic impact on growth factor and binding protein levels. OUP Academic
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1 review for Toremifene Citrate 60mg for scientific research
What are Toremifene Citrate Tablets used for in research?
Toremifene Citrate Tablets are used for in vitro receptor-binding studies, transcriptional pathway analysis, and mechanistic molecular research. They are suitable for multi-omic and computational studies requiring high-purity SERM reagents.
Are these tablets suitable for in vivo experiments?
No. These tablets are intended strictly for laboratory research, including biochemical, cellular, and computational assays. They are not for human or animal use.
How should I store Toremifene Citrate Tablets?
Store in a sealed, moisture-free container, away from direct light, at controlled room temperature (20–25°C). Proper storage preserves stability and research-grade purity.
Can the tablets be split or crushed for solution preparation?
Yes, tablets can be split or powdered under a fume hood. Ensure complete homogenization and minimal dust exposure to maintain experimental accuracy.
What solvents are compatible for tablet dissolution?
Analytical-grade aqueous buffers or organic solvents (e.g., PBS, DMSO) are compatible for in vitro assay preparation. Ensure complete dissolution for consistent ligand-binding studies.
How is the purity of Toremifene Citrate Tablets verified?
Purity is confirmed by HPLC analysis, structural verification, and contaminant screening. Each batch undergoes strict quality control to ensure reproducible results.
Can Toremifene Citrate Tablets be used in multi-omic workflows?
Yes. They are ideal for proteomics, transcriptomics, metabolomics, and chromatin studies, enabling integration with computational modeling and pathway mapping.
Are these tablets suitable for high-throughput screening?
Yes. Their consistent composition and tablet uniformity make them suitable for high-throughput receptor or pathway assays in controlled in vitro settings.
Is the tablet form preferable to powder for research use?
Yes. Tablets provide mass accuracy, reduced handling variability, and stable formulation, which improves reproducibility across experiments.
Do the tablets degrade over time?
When stored properly in dry, light-protected conditions, Toremifene Citrate Tablets remain stable for research use. Avoid prolonged exposure to moisture or high temperatures.
Can the tablets be used for receptor-binding kinetics studies?
Yes. They are widely used in ligand-receptor interaction and binding affinity assays, providing high-purity SERM for reproducible measurements.
Are there any special handling precautions?
Always wear gloves, lab coat, and protective eyewear. Use a fume hood when grinding or preparing suspensions to minimize dust exposure.
Is the product batch consistent for large-scale research?
Yes. Manufactured under factory-controlled conditions, bulk and wholesale supply ensure consistent purity, tablet uniformity, and reproducible experimental data.
Can Toremifene Citrate Tablets be integrated into computational studies?
Yes. Experimental data derived from these tablets can be used for docking simulations, molecular dynamics, and receptor-ligand interaction modeling.














mood –
The package is damaged, you need to resend it to me, thank you