No products in the cart.
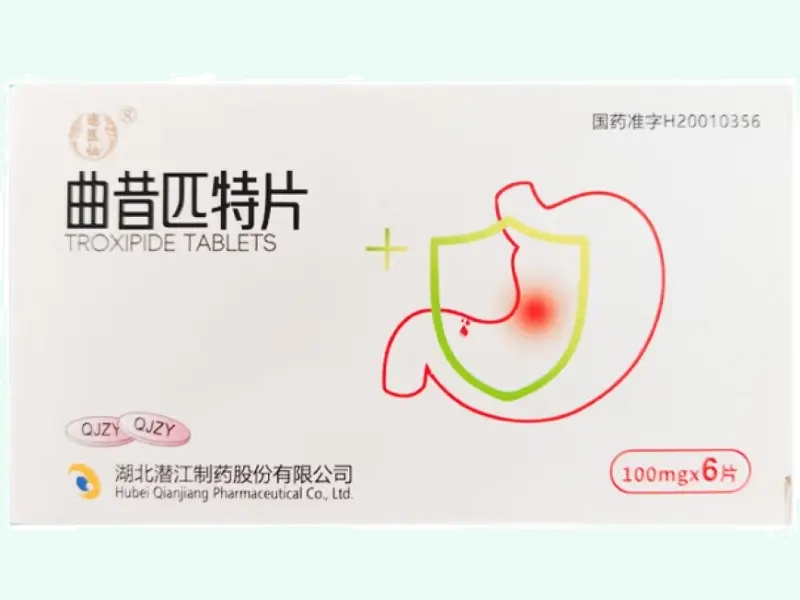
Troxipide Tablets 100?mg ×?6 Tablets (Miaoyixian) Wholesale price comparison
$1.00
Troxipide Tablets (brand: Miaoyixian) are orally administered anti-ulcer agents primarily used in experimental models of gastric mucosal injury, gastritis, and NSAID-induced ulcers. Each box contains 6 tablets of 100?mg Troxipide. Manufactured by Hubei Qianjiang Pharmaceutical Co., Ltd., this product is registered under China NMPA H20010356.?? Research-use only.?Please consult staff for other specifications and uses?
Description
Troxipide Tablets Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Troxipide Tablets (Miaoyixian) |
| Generic Name | Troxipide |
| Formulation | Film-coated tablet |
| Strength | 100?mg per tablet |
| Pack Size | 6 tablets (1 blister) per box |
| Dosage Form | Tablet |
| Approval Number | H20010356 (NMPA, China) |
| Product Code | 86901840000888 |
| Manufacturer | Hubei Qianjiang Pharmaceutical Co., Ltd. |
| Barcode | 6906291004083 |
| CAS Number | 10405-02-4 |
| Molecular Formula | C15H15NO3 |
| Storage Conditions | Store in a cool, dry place below 25?°C |
| Intended Use | For laboratory research use only |
Troxipide Tablets Mechanism of Action
Troxipide enhances gastric mucosal defense by increasing mucus secretion, improving microcirculation, and suppressing inflammatory cytokines. It does not affect acid secretion, making it suitable for combination research with PPIs or H2-blockers.
It may inhibit neutrophil adhesion, oxidative stress, and prostaglandin E2 (PGE2) degradation, promoting epithelial healing in ulcer models.
Troxipide Tablets Research Applications
NSAID-induced ulcer models
Gastritis and mucosal damage studies
Oxidative stress and GI inflammation assays
Combination studies with PPIs (e.g., Omeprazole)
In vivo animal models for gastric protection
In vitro studies on epithelial cell regeneration
Safety Profile & Handling Instructions
Observed Effects in Preclinical Settings:
Mild GI discomfort (rare)
No significant hepatotoxicity or nephrotoxicity
Well-tolerated in rodent models
Handling Recommendations:
Use lab gloves, mask, and eye protection
Avoid skin or eye contact
Not for inhalation or ingestion
Dispose according to chemical waste protocols
?? Research-Use Disclaimer
This product is designated exclusively for laboratory research use. It is not approved for clinical diagnosis, treatment, or veterinary applications. Any misuse may pose significant health risks. Handle in compliance with local regulations and lab safety standards.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 20 × 16 × 20 cm |











Reviews
There are no reviews yet.