No products in the cart.
Sale
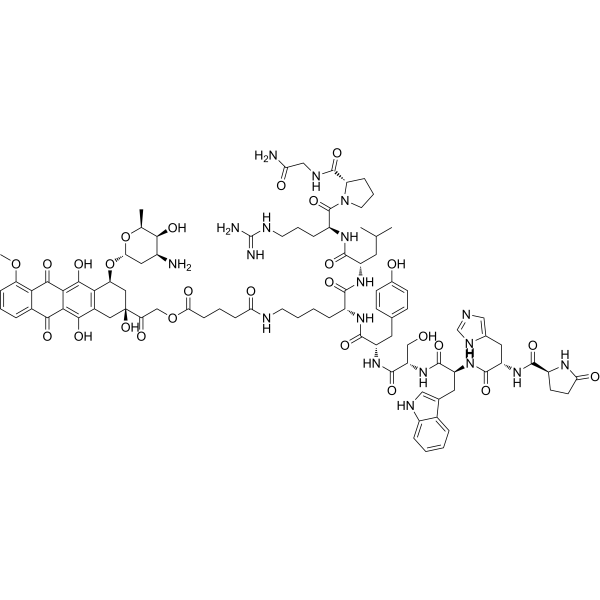
Zoptarelin Doxorubicin (CAS No. 139570-93-7)
Original price was: $22.00.$17.00Current price is: $17.00.
Zoptarelin doxorubicin (AEZS-108; AN-152) is a targeted hybrid anticancer peptide-drug conjugate composed of a luteinizing hormone-releasing hormone analogue linked to doxorubicin. It selectively targets tumors expressing LHRH receptors, induces apoptosis, and inhibits tumor progression. For research use only.
Description
Product Description
Zoptarelin doxorubicin (CAS No. 139570-93-7), also known as AEZS-108 or AN-152, represents an advanced hybrid anticancer agent specifically designed to improve the therapeutic index of traditional chemotherapy. This innovative compound is a peptide-drug conjugate that combines the selective targeting capability of a luteinizing hormone-releasing hormone (LHRH) analogue with the potent cytotoxic activity of the chemotherapeutic drug doxorubicin. By integrating these two components into a single hybrid molecule, Zoptarelin doxorubicin offers a research model for exploring how selective drug delivery can maximize tumor suppression while minimizing systemic toxicity.
The rationale behind the development of Zoptarelin doxorubicin arises from the fact that many malignant tumors, including ovarian, endometrial, breast, and prostate cancers, frequently overexpress receptors for LHRH. These receptors, normally involved in the regulation of reproductive hormones, become aberrantly expressed on tumor cells, making them attractive targets for drug delivery. By coupling doxorubicin, a powerful anthracycline chemotherapy agent, to an LHRH analogue, researchers can direct the cytotoxic effects of the drug specifically to tumor sites, sparing normal tissues that lack receptor expression.
Traditional doxorubicin therapy is effective but limited by severe systemic side effects, such as cardiotoxicity, myelosuppression, and gastrointestinal toxicity. Zoptarelin doxorubicin addresses this challenge by exploiting receptor-mediated endocytosis. When the hybrid molecule binds to LHRH receptors on the surface of tumor cells, the complex is internalized, releasing doxorubicin inside the cancer cell where it can intercalate into DNA, inhibit topoisomerase II, and generate free radicals that damage cellular structures. This targeted approach not only increases the intracellular concentration of the drug in tumor cells but also decreases off-target toxicity.
In vitro and preclinical studies have demonstrated that Zoptarelin doxorubicin effectively abolishes tumor progression and induces remarkable apoptosis in LHRH receptor-positive cancer cells. These effects highlight its potential value in antineoplastic research, particularly for hormone-dependent cancers. Furthermore, because its mechanism depends on receptor targeting, Zoptarelin doxorubicin can serve as an important model for studying precision oncology and the development of future ligand-drug conjugates.
Beyond direct cytotoxicity, Zoptarelin doxorubicin provides opportunities for investigating the interplay between chemotherapy and the tumor microenvironment. By selectively delivering doxorubicin to tumors, researchers can analyze how reduced systemic exposure alters immune responses, angiogenesis, and tumor metabolism. In addition, the hybrid structure of Zoptarelin doxorubicin allows comparative studies against unconjugated doxorubicin, providing insights into how receptor targeting modifies pharmacokinetics, biodistribution, and therapeutic outcomes.
Another important research application of Zoptarelin doxorubicin is in drug resistance studies. Many cancers eventually develop resistance to anthracycline-based therapies. By targeting delivery through LHRH receptors, Zoptarelin doxorubicin may bypass some resistance mechanisms, such as efflux pumps, while also enabling investigation into new resistance pathways that emerge in receptor-positive tumors.
In summary, Zoptarelin doxorubicin represents a paradigm shift in anticancer drug design, where receptor targeting is combined with cytotoxic potency to produce a compound with high research value. Its ability to induce apoptosis, suppress tumor growth, and minimize systemic toxicity makes it a promising candidate for the study of targeted therapies, tumor biology, and receptor-mediated drug delivery systems.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Zoptarelin doxorubicin |
| CAS Number | 139570-93-7 |
| Synonyms | AEZS-108, AN-152 |
| Category | Hybrid peptide-drug conjugate, anticancer research agent |
| Application | Targeted antineoplastic research, receptor-positive tumor studies |
| Appearance | Red to orange crystalline powder (doxorubicin-linked conjugate) |
| Purity | ≥95% (HPLC verified) |
| Molecular Formula | C73H96N14O14 (approx., doxorubicin conjugated peptide) |
| Molecular Weight | ~1369.64 g/mol |
| Sequence | LHRH analogue conjugated to doxorubicin |
| Solubility | Soluble in DMSO, ethanol, slightly soluble in water |
| Stability | Stable as lyophilized powder at -20°C |
| Storage | Store at -20°C, protected from light |
| Mechanism | LHRH receptor-mediated internalization and doxorubicin release |
| Biological Activity | Tumor growth inhibition, apoptosis induction in vitro |
| Research Use | Cancer immunology, peptide-drug conjugates, precision oncology |
| Safety Note | For research use only, not for clinical or veterinary applications |
Detailed Specifications Commentary
Zoptarelin doxorubicin is synthesized as a high-purity peptide-drug conjugate and undergoes rigorous quality control testing, including HPLC and mass spectrometry verification. Its stability as a lyophilized powder makes it suitable for long-term storage, though light protection is essential due to the photosensitivity of doxorubicin.
Its solubility profile allows for flexibility in experimental setups, with DMSO and ethanol being the most effective solvents for stock solutions. Once reconstituted, Zoptarelin doxorubicin should be used promptly to ensure biological activity.
The conjugate’s approximate molecular weight reflects the combination of the peptide targeting moiety and doxorubicin payload. Importantly, this hybrid structure enables receptor targeting and controlled intracellular release of the cytotoxic agent, which distinguishes it from unconjugated chemotherapy drugs.
Zoptarelin doxorubicin is most often used in studies of receptor-positive tumors, where its selective delivery can be directly compared to traditional chemotherapy. It is especially useful in models of ovarian, breast, and endometrial cancers, as well as other malignancies that express LHRH receptors.
Mechanism of Action & Research Applications
Mechanism of Action
The hybrid structure of Zoptarelin doxorubicin ensures dual functionality: receptor targeting and cytotoxicity. The LHRH analogue portion binds with high affinity to LHRH receptors on tumor cells. Upon receptor binding, the entire conjugate is internalized into the cell through receptor-mediated endocytosis. Once inside, enzymatic processes release doxorubicin from the conjugate, allowing it to exert its well-documented cytotoxic effects.
DNA Intercalation: Doxorubicin intercalates between DNA base pairs, preventing replication.
Topoisomerase II Inhibition: Blocks the enzyme responsible for relieving torsional strain during DNA replication.
Reactive Oxygen Species (ROS): Generates free radicals that damage cellular membranes, proteins, and DNA.
Apoptosis Induction: Activates caspase pathways and leads to programmed cell death.
Research Applications
Targeted Chemotherapy Studies
Zoptarelin doxorubicin provides a research model for testing how ligand-drug conjugates improve chemotherapy selectivity. By focusing drug activity on receptor-positive tumors, researchers can analyze tumor suppression with reduced systemic toxicity.LHRH Receptor Biology
Since many tumors express LHRH receptors, this conjugate is a valuable tool for mapping receptor distribution, density, and role in cancer progression.Comparative Oncology
Enables direct comparisons between unconjugated doxorubicin and receptor-targeted conjugates to determine differences in efficacy and toxicity.Resistance Mechanism Studies
Investigating whether targeted delivery can overcome traditional anthracycline resistance in tumor cells.Tumor Microenvironment Research
By reducing systemic exposure, researchers can study how targeted therapy influences angiogenesis, immune modulation, and tumor stroma interactions.Drug Development Platform
Serves as a prototype for future peptide-drug conjugates, guiding the design of other receptor-targeted chemotherapeutics.
Side Effects
Since Zoptarelin doxorubicin is intended strictly for research purposes, no approved clinical side effect profile is established. However, research studies and comparisons to unconjugated doxorubicin provide insights into potential effects.
Potential Preclinical Observations
Reduced Cardiotoxicity: Compared to free doxorubicin, receptor targeting may lower cardiotoxicity, a common dose-limiting factor.
Lower Myelosuppression: Selective tumor delivery could reduce bone marrow toxicity, though further validation is required.
Localized Cytotoxicity: Effective apoptosis is observed in receptor-positive cells, with reduced impact on receptor-negative tissues.
Off-Target Risks: In cases where LHRH receptors are expressed in normal tissues, some cytotoxicity may still occur.
Cumulative Toxicity: As with all anthracyclines, long-term exposure may present cumulative toxicity risks.
Experimental Safety Considerations
Dosing studies are needed to balance therapeutic efficacy and safety.
Careful handling is required due to the potent cytotoxic properties of doxorubicin.
Research designs should monitor cell viability, apoptosis, and systemic toxicity in preclinical models.
Overall, while Zoptarelin doxorubicin shows potential for reduced systemic toxicity, researchers must remain cautious and recognize that receptor expression patterns can influence selectivity.
Disclaimer
For laboratory research use only. Not for human or veterinary applications.
Keywords
Zoptarelin doxorubicin, AEZS-108, AN-152, CAS 139570-93-7, LHRH-targeted chemotherapy, peptide-drug conjugate, receptor-positive tumor research, hybrid anticancer peptide, targeted doxorubicin conjugate, cancer apoptosis peptide
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 52 × 28 × 52 cm |
What is Zoptarelin doxorubicin?
A hybrid anticancer peptide-drug conjugate combining an LHRH analogue with doxorubicin.
What is its CAS number?
CAS No. 139570-93-7.
What are its synonyms?
AEZS-108 and AN-152.
How does it work?
It binds to LHRH receptors, is internalized into tumor cells, and releases doxorubicin to induce apoptosis.
What cancers can it target in research?
Ovarian, endometrial, breast, and prostate cancers with LHRH receptor expression.
Why is it considered targeted therapy?
Because it delivers cytotoxic doxorubicin selectively to receptor-positive tumors.
What makes it better than free doxorubicin?
Potential for reduced systemic toxicity and enhanced tumor selectivity.
Is it soluble in water?
Slightly soluble; better solubility in DMSO or ethanol.
Can it be used clinically?
No, it is for research use only.
What is its storage condition?
Store lyophilized at -20°C, protected from light.
















Reviews
There are no reviews yet.